��Ŀ����
2��ç���ᣨC7H10O5������Ч�Ը�������H5N1�������в�����ҩ���ơ�����Ҫ�ɷ֣����ɴ���ҩ�˽���������ȡ�õ�����ͼ����ç����Ϊԭ�Ϻϳ�ijЩ���ʵ�·�ߣ�
������ͼ�ش��������⣺
��1��A�к��е����ֺ��������ŷֱ����Ȼ����ǻ�
��2����֪ϩ��ʽ�ṹ��C=C-OH�������ȶ����ڡ���ç����Ľṹ��ʽΪ
 ��
����3��ç�����C�ķ�Ӧ��������ȥ��Ӧ��
��4��д��C��D�Ļ�ѧ��Ӧ����ʽ
 +CH3CH2OH$��_{��}^{Ũ����}$H20+
+CH3CH2OH$��_{��}^{Ũ����}$H20+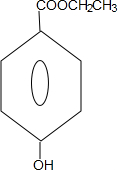 ��
����5����C������ͬ�����ŵ�ͬ���칹�壨��C������3�֣�
���� �ݷ�Ӧ������B�Ľṹ��ʽ��֪����A��B��������������Ӧ����AΪ ��ϩ��ʽ�ṹ��C=C-OH�������ȶ����ڣ�ç����ķ���ʽΪC7H10O5��ç����Ϊ
��ϩ��ʽ�ṹ��C=C-OH�������ȶ����ڣ�ç����ķ���ʽΪC7H10O5��ç����Ϊ ��ç������һ��������ʧˮ���ɵ�C���Ȼ�������ɫ��˵�����з��ǻ���CΪ
��ç������һ��������ʧˮ���ɵ�C���Ȼ�������ɫ��˵�����з��ǻ���CΪ �����ǻ����������Ҵ�����������Ӧ����D��DΪ
�����ǻ����������Ҵ�����������Ӧ����D��DΪ ���ݴ˷�����
���ݴ˷�����
��� �⣺��1���ݷ�Ӧ������B�Ľṹ��ʽ��֪����A��B��������������Ӧ����AΪ �������Ȼ����ǻ����ʴ�Ϊ���Ȼ����ǻ���
�������Ȼ����ǻ����ʴ�Ϊ���Ȼ����ǻ���
��2��ϩ��ʽ�ṹ��C=C-OH�������ȶ����ڣ�ç����ķ���ʽΪC7H10O5��ç����Ϊ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3��ç������һ��������ʧˮ���ɵ�C���Ȼ�������ɫ��˵�����з��ǻ���CΪ ��ç�����C�ķ�Ӧ��������ȥ��Ӧ���ʴ�Ϊ����ȥ��Ӧ��
��ç�����C�ķ�Ӧ��������ȥ��Ӧ���ʴ�Ϊ����ȥ��Ӧ��
��4��CΪ �����ǻ����������Ҵ�����������Ӧ����D��DΪ
�����ǻ����������Ҵ�����������Ӧ����D��DΪ ����ѧ����ʽΪ
����ѧ����ʽΪ +CH3CH2OH$��_{��}^{Ũ����}$H20+
+CH3CH2OH$��_{��}^{Ũ����}$H20+ ���ʴ�Ϊ��
���ʴ�Ϊ�� +CH3CH2OH$��_{��}^{Ũ����}$H20+
+CH3CH2OH$��_{��}^{Ũ����}$H20+ ��
��
��5��ç������һ��������ʧˮ���ɵ�C���Ȼ�������ɫ��˵�����з��ǻ���CΪ ����C������ͬ�����ŵ�ͬ���칹�����ڼ��3�֣��ʴ�Ϊ��3��
����C������ͬ�����ŵ�ͬ���칹�����ڼ��3�֣��ʴ�Ϊ��3��
���� ���⿼���л�����ƶϣ�ע��B�Ľṹ����ת����������Ϊ����Ĺؼ����ѶȽϴ�

 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�| A�� | �� | B�� | þ | C�� | Һ�� | D�� | ��ˮ |
| A�� | ����ʹʯ����ֽ����ɫ����Һ�У�Na+��AlO2-��S2-��SO42- | |
| B�� | ��ˮ�����H+Ũ��c��H+��=10-12mol•L-1����Һ�У�Cl-��CO32-��NH4+��SO32- | |
| C�� | �ڼ��������ܲ���H2����Һ�У�NH4 +��Na+��Fe2+��NO3- | |
| D�� | pH=2����Һ�У�Na+��SO42-��NO3 -��CO32- |
| A�� | ����������Ư��������������Ư����Һ�ļ��� | |
| B�� | ���ȷ�Ӧ�ǹ�ҵ�������õķ��� | |
| C�� | �����ڳ�ʪ�Ŀ����������⣬�为����ӦʽΪ2H2O+02+4e-�T4OH- | |
| D�� | ʵ���Ҵ�������Ż�ʱ��������ɰ�Ӹ��� |
| A�� | �к͵�����������ʵ���Ũ������ʹ�����Һ����������NaOH��Һ���ڴ��� | |
| B�� | �����£�20 LpH=12��Na2CO3��Һ�к��е�OH-������Ϊ0.2NA | |
| C�� | ��0.1 mol/LCH3COOH��Һ�м�������CH3COONa�� �壬��Һ��$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$ ���� | |
| D�� | һ���¶��£�10mL 0.50mol•L-1NH4Cl��Һ��20mL 0.25mol•L-1NH4C1��Һ��NH4+���ʵ�����ͬ |
| A�� | һ���ǽ���Ԫ�� | |
| B�� | һ���Ƿǽ���Ԫ�� | |
| C�� | �����ǽ���Ԫ�أ�Ҳ�����Ƿǽ���Ԫ�� | |
| D�� | ���Ͼ�����ȷ |
| A�� | ʹ��̪���ɫ����Һ�У�Na+��Al3+��SO42-��Cl- | |
| B�� | $\frac{{K}_{w}}{c��{H}^{+}��}$=1��10-13mol•L-1����Һ�У�NH4+��Ca2+��Cl-��NO3- | |
| C�� | ��Al��Ӧ�ܷų�H2����Һ�У�Fe2+��K+��NO3-��SO42- | |
| D�� | ˮ�����c��H+��=1��10-13mol•L-1����Һ�У�K+��Na+��AlO2-��CO32- |