��Ŀ����
15��A��B��C��D���ֶ�����Ԫ�أ�0.5molA��Ԫ�ص����ӵõ�NA�����Ӻ�ԭΪ����ԭ�ӣ�0.4gA��������ǡ����100mL0.2mol/L��������ȫ��Ӧ��AԪ��ԭ�Ӻ�������������������ȣ�BԪ��ԭ�Ӻ����������AԪ��ԭ�Ӻ����������1��C�����Ӻ�����Ӳ�����AԪ�ص����Ӻ�����Ӳ�����1��DԪ��ԭ�������������Ǵ�����������2��������д���пո���1���ƶ�A��B��C��D����Ԫ�صķ���AMg��BAl��CCl��DC��
��2��C��һ�������ӵĽṹʾ��ͼ
 ��
����3��DԪ�ص����������Ľṹʽ��O=C=O��
��4��C��D��Ԫ���γɵĻ��������ʽ
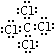 �������ں��м��Լ���
�������ں��м��Լ���
���� ������Ԫ��A��B��C��D�У�0.5mol AԪ�ص����ӵõ�NA�����ӱ���ԭΪ����ԭ�ӣ���A����Ϊ��2����λ����ɵ������ӣ�0.4g A��������ǡ����100ml 0.2mol/L��������ȫ��Ӧ����AO+2HCl�TACl2+H2O����֪M��AO��=$\frac{0.4g}{0.01mol}$=40g/mol������A��Ħ������Ϊ40g/mol-16g/mol=24g/mol����Aԭ�Ӻ���������Ŀ��������Ŀ��ȣ���������Ϊ12����AΪMgԪ�أ�BԪ��ԭ�Ӻ����������AԪ��ԭ�Ӻ����������1����BΪAl��C�����Ӻ�����Ӳ�����AԪ�ص����Ӻ�����Ӳ�����1��C�����Ӻ��������Ϊ18����CΪClԪ�أ�DԪ��ԭ�����������������ڲ��������2����ԭ��ֻ����2�����Ӳ㣬����������Ϊ4����DΪCԪ�أ��ݴ˽��
��� �⣺������Ԫ��A��B��C��D�У�0.5mol AԪ�ص����ӵõ�NA�����ӱ���ԭΪ����ԭ�ӣ���A����Ϊ��2����λ����ɵ������ӣ�0.4g A��������ǡ����100ml 0.2mol/L��������ȫ��Ӧ����AO+2HCl�TACl2+H2O����֪M��AO��=$\frac{0.4g}{0.01mol}$=40g/mol������A��Ħ������Ϊ40g/mol-16g/mol=24g/mol����Aԭ�Ӻ���������Ŀ��������Ŀ��ȣ���������Ϊ12����AΪMgԪ�أ�BԪ��ԭ�Ӻ����������AԪ��ԭ�Ӻ����������1����BΪAl��C�����Ӻ�����Ӳ�����AԪ�ص����Ӻ�����Ӳ�����1��C�����Ӻ��������Ϊ18����CΪClԪ�أ�DԪ��ԭ�����������������ڲ��������2����ԭ��ֻ����2�����Ӳ㣬����������Ϊ4����DΪCԪ�أ�
��1��������������֪��A��B��C��D����Ԫ�ط��ŷֱ�Ϊ��Mg��Al��Cl��C��
�ʴ�Ϊ��Mg��Al��Cl��C��
��2��Cl�����ӵĽṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3��DԪ�ص����������ΪCO2���ṹʽ��O=C=O���ʴ�Ϊ��O=C=O��
��4��C��D��Ԫ���γɵĻ�����ΪCCl4������ʽΪ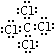 �������ں��м��Լ����ʴ�Ϊ��
�������ں��м��Լ����ʴ�Ϊ��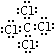 �����ԣ�
�����ԣ�
���� ���⿼��ṹ����λ�ù�ϵӦ�ã����ضԻ�ѧ����Ŀ��飬�ƶ�Ԫ���ǽ���ؼ���

 ��ǰ����ϵ�д�
��ǰ����ϵ�д�| A�� | ij���ʵ�ˮ��ҺPH��7���������һ�������ǿ�������� | |
| B�� | pH=4.5�ķ���֭��c��H+����pH=6.5��ţ����c��H+����100�� | |
| C�� | ϡ�ʹ�����Һ����Һ���������ӵ�Ũ�Ⱦ����� | |
| D�� | pH=5.6��CH3COOH��CH3COONa�����Һ�У�c��Na+����c��CH3COO-�� |
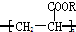 �������й�����˵���У�����ȷ���ǣ�������
�������й�����˵���У�����ȷ���ǣ�������| A�� | ������CH2=CH-COOR���ӳɾۺϷ�Ӧ�õ� | |
| B�� | ��һ���������ܷ����ӳɷ�Ӧ | |
| C�� | �۱�ϩ����������һ�ֻ���� | |
| D�� | �۱�ϩ�����Ǹ߷��ӻ����� |
���з�������Ҫ����ǣ�������
| A�� | ֻ�Т� | B�� | ֻ�Тڢ� | C�� | ֻ�Тڢۢ� | D�� | ֻ�Т٢ڢ� |
| Ԫ�ر�� | Ԫ��������ԭ�ӣ�����ӣ��ṹ |
| T | �����������Ǵ�����������3�� |
| X | �����µ���Ϊ˫ԭ�ӷ��ӣ������к���3�Թ��õ��Ӷ� |
| Y | M���K����1������ |
| Z | ��������Ԫ�صļ������а뾶��С |
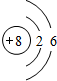 ��
����2��Ԫ��Y��Ԫ��Z��ȣ������Խ�ǿ����Na����Ԫ�ط��ű�ʾ�������б�������֤����һ��ʵ����cd������ţ���
a��Y���ʵ��۵��Z���ʵ� b��Y�Ļ��ϼ۱�Z��
c��Y������ˮ��Ӧ��Z���ʾ��� d��Y����������Ӧ��ˮ����ļ��Ա�Zǿ
��3��д��T��Y�γɵĻ�����Ļ�ѧʽNa2O��Na2O2��
��4��Ԫ��T����Ԫ����ԭ�Ӹ�����1��1�����γɻ�����Q��Ԫ��X����Ԫ����ԭ�Ӹ�����1��2�����γɳ��������ȼ�ϵĻ�����W��Q��W����������ԭ��Ӧ������X���ʺ�T����һ���⻯�д���÷�Ӧ�Ļ�ѧ����ʽ��N2H4+2H2O2�TN2��+4H2O��
| A�� | ���ϵ����⻯����۷е������� | |
| B�� | ���ϵ��µ��ʵĻ�ԭ������ | |
| C�� | ���ϵ�������������ˮ������������ | |
| D�� | ���ϵ���ԭ�ӵõ��ӵ���������ǿ |
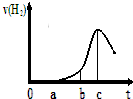 ij��ȤС����С����ý������ᷴӦ����ʵ�飬��5.4g����ƬͶ��500mL 0.5mol•L-1��������Һ�У���ͼΪ��Ӧ�������������뷴Ӧʱ��Ĺ�ϵͼ��
ij��ȤС����С����ý������ᷴӦ����ʵ�飬��5.4g����ƬͶ��500mL 0.5mol•L-1��������Һ�У���ͼΪ��Ӧ�������������뷴Ӧʱ��Ĺ�ϵͼ��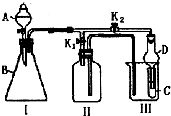 ij��ȤС���������ͼ��ʾ��ʵ��װ�ã��ȿ�������ȡ���壬�ֿ�������֤���ʵ����ʣ�
ij��ȤС���������ͼ��ʾ��ʵ��װ�ã��ȿ�������ȡ���壬�ֿ�������֤���ʵ����ʣ� ����A��B��C���ֶ�����Ԫ�أ�ԭ���������ε�����A��C��������֮��Ϊ27������������֮��Ϊ5��0.9g����B���������ᷴӦ���ռ�������1.12L����״��������ش��������⣺
����A��B��C���ֶ�����Ԫ�أ�ԭ���������ε�����A��C��������֮��Ϊ27������������֮��Ϊ5��0.9g����B���������ᷴӦ���ռ�������1.12L����״��������ش��������⣺