��Ŀ����
1����������ƣ�Na2S2O3•5H2O�����������մ��ֳ�Ϊ��������������������ҵ�����ͼ���Ҳ������ֽ��Ư��������������������ˮ���������Ҵ������ȡ�������ֱ棬��ҵ�ϳ����������Ʒ�������Ʊ���ijʵ��ģ�ҵ���ȡ��������ƣ��䷴Ӧװ�ü������Լ���ͼ1��
ʵ������������Ϊ��
�ٿ�����Һ©����ʹ�����������£��ʵ����ڷ�Һ�ĵ��٣�ʹ��Ӧ������SO2������ȵ�ͨ��Na2S��Na2CO3�Ļ����Һ�У�ͬʱ�����綯������������ˮԡ���ȣ���
��ֱ���۳��Ļ��Dz�����ʧ����������Һ��PH�ӽ�7ʱ��ֹͣͨ��SO2����
�۳��ȹ��ˣ�����Һ����Ũ������ȴ����Na2S2O3•5H2O
���ھ����ˡ�ϴ�ӡ�����õ������Ʒ
��1��д������A������������ƿ���������ϴ��ʱ��Ϊ�˼��ٲ������ʧ���Լ��������Ҵ�
��2��Ϊ�˱�֤��������ƵIJ�����ʵ���в�������ҺPH��7���������ӷ���ʽ����ԭ��S2O32-+2H+=S��+H2O+SO2��
��3��д��������ƿB����ȡNa2S2O3��Ӧ���ܻ�ѧ����ʽ4SO2+2Na2S+Na2CO3=3Na2S2O3+CO2
��4�����õ��IJ�Ʒ���ܺ��з�ӦNa2SO4���ʣ������ʵ�����Ʒ���Ƿ����Na2SO4����Ҫ˵��ʵ�����������ͽ���ȡ������Ʒ��������ϡ���ᡢ���á�ȡ�ϲ���Һ������ˣ�ȡ��Һ�����μ�BaCl2��Һ�������ֳ�����˵������Na2SO4����
��5���ⶨ��Ʒ����
ȷ��ȡ1.00g��Ʒ������������ˮ�ܽ⣬�Ե�����ָʾ����0.1000mol•L-1��ı���Һ�ζ�����Ӧԭ��Ϊ2S2O32-+I2=S4O33-+2I-���ζ���ʼ���յ��Һ��λ����ͼ2�����ĵ�ı���Һ���Ϊ16.00mL����Ʒ�Ĵ���Ϊ79.36%
��6��Na2S2O3������������������Һ�ױ�Cl2����SO42-���÷�Ӧ�����ӷ���ʽΪS2O32?+4Cl2+5H2O=2SO42?+8Cl?+10H+��
���� ��1���������ṹ��������֪AΪ������ƿ��
����Ŀ��Ϣ��֪�����������������ˮ���������Ҵ���Ӧ���Ҵ�ϴ�ӣ��������ܽ����ʧ��
��2��Na2S2O3�����������»ᷴӦ����S�Ͷ�������
��3��������ƿ��SO2��Na2S��Na2CO3��Ӧ����Na2S2O3��ͬʱ���ɶ�����̼��
��4���������ᣬNa2S2O3��Ӧ����S���������ú�ȡ�ϲ���Һ���μ�BaCl2��Һ��������Һ���Ƿ�����������ӣ�
��5������ͼʾ�ĵζ�����Һ��������������ն�����Ȼ���������ĵ�ı���Һ��������ݷ�Ӧ2S2O32-+I2�TS4O62-+2I-��֪��n��S2O32-��=2n��I2����Ȼ��ⵥ�ʵ����ʵ��������Na2S2O3•5H2O��������Ʒ�Ĵ��ȣ�
��6��Na2S2O3��ԭ�Խ�ǿ������Һ���ױ�Cl2������SO42-����Ӧ���������ơ��Ȼ��ƣ���ƽ��д���ӷ���ʽ��
��� �⣺��1���������ṹ��������֪AΪ������ƿ������Ŀ��Ϣ��֪�����������������ˮ���������Ҵ���Ӧ���Ҵ�ϴ�ӣ��������ܽ����ʧ��
�ʴ�Ϊ��������ƿ���Ҵ���
��2��Na2S2O3�����������»�����S�Ͷ��������ʻ��½����䷴Ӧ�����ӷ���ʽΪ��S2O32-+2H+=S��+H2O+SO2����
�ʴ�Ϊ��S2O32-+2H+=S��+H2O+SO2����
��3��������ƿ��SO2��Na2S��Na2CO3��Ӧ����Na2S2O3��ͬʱ���ɶ�����̼����Ӧ�ܷ���ʽΪ��4SO2+2Na2S+Na2CO3=3Na2S2O3+CO2��
�ʴ�Ϊ��4SO2+2Na2S+Na2CO3=3Na2S2O3+CO2��
��4������Ʒ���Ƿ����Na2SO4��ʵ�鷽��Ϊ��ȡ������Ʒ��������ϡ���ᡢ���á�ȡ�ϲ���Һ������ˣ�ȡ��Һ�����μ�BaCl2��Һ�������ֳ�����˵������Na2SO4���ʣ�
�ʴ�Ϊ��ȡ������Ʒ��������ϡ���ᡢ���á�ȡ�ϲ���Һ������ˣ�ȡ��Һ�����μ�BaCl2��Һ�������ֳ�����˵������Na2SO4���ʣ�
��5������ͼʾ�ĵζ�����Һ���֪���ζ����г�ʼ����Ϊ2.50mL���ζ��յ�Һ�����Ϊ18.50mL���������ĵ�ı���Һ���Ϊ18.50mL-2.50mL=16.00mL��
���ݷ�Ӧ2S2O32-+I2�TS4O62-+2I-��֪��n��S2O32-��=2n��I2��������1.00 g��Ʒ�к���Na2S2O3•5H2O����Ϊ��0.1000 mol•L-1��16.00��10-3L��2��248g/mol=0.7936g�������Ʒ�Ĵ���Ϊ��$\frac{0.7936g}{1.00g}$��100%=79.36%��
�ʴ�Ϊ��16.00��79.36��
��6��Na2S2O3��ԭ�Խ�ǿ������Һ���ױ�Cl2������SO42-����ƽ������ӷ���ʽΪ��S2O32?+4Cl2+5H2O=2SO42?+8Cl?+10H+��
�ʴ�Ϊ��S2O32?+4Cl2+5H2O=2SO42?+8Cl?+10H+��
���� ���⿼��ʵ�鷽���������漰����ʶ�𡢶Բ����ķ������ۡ�����ʽ��д�����Ӽ��顢��ѧ����ȣ�����ʵ�����������֪ʶ�ۺ�Ӧ�������Ŀ��飬��Ŀ�Ѷ��еȣ�

 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�| A�� | ����ʹ�õ綯�� | |
| B�� | ����̫���ܡ����ܺ����ܵ���Դ���滯ʯȼ�� | |
| C�� | ����˽�˹����ʹ���������湫���� | |
| D�� | ��ú��ȼ����Ϊ��Ҫ����ȼ�� |
| A�� | ��������ΪNA��NO2��CO2�Ļ�������к��е���ԭ������2NA | |
| B�� | 28g��ϩ�ͻ����飨C4H8���Ļ�������к��е�̼ԭ����Ϊ2NA | |
| C�� | ���³�ѹ�£�92g��NO2��N2O4������庬�е�ԭ����Ϊ6NA | |
| D�� | ���³�ѹ�£�22.4L����������þ�۳�ַ�Ӧ��ת�Ƶĵ�����Ϊ2NA |
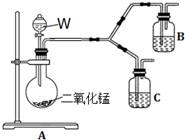 �±���ijѧ��Ϊ̽��AgCl����ת��ΪAg2S�����ķ�Ӧ����ʵ��ļ�¼��
�±���ijѧ��Ϊ̽��AgCl����ת��ΪAg2S�����ķ�Ӧ����ʵ��ļ�¼��| �� �� | �� �� |
| ��ȡ5mL 0.1mol/L AgNO3��һ�����0.1mol/L NaCl��Һ����ϣ��� | ����������ɫ���� |
| ������������Һ�м���2.5mL 0.1mol/L Na2S��Һ�� | ����Ѹ�ٱ�Ϊ��ɫ |
| ��������ɫ��Һ�������ڿ����У����Ͻ��裮 | �ϳ�ʱ�������Ϊ���ɫ |
| �����˳����е����ɫ��������������HNO3��Һ�� | ��������ɫ���壬���������ܽ� |
| �������˵õ���ҺX�Ͱ�ɫ����Y����X�еμ�Ba��NO3��2��Һ�� | ������ɫ���� |
��2����֪��25��ʱKsp��AgCl��=1.8��10-10��Ksp��Ag2S��=6��10-30���˳���ת����Ӧ��ƽ�ⳣ��K=5.4��109��
��3������V�в����İ�ɫ�����Ļ�ѧʽΪBaSO4������������ɫ����������AgCl�⣬������S��
��4��Ϊ�˽�һ��ȷ�ϲ���������ɫ����������ԭ�����������ͼ��ʾ�ĶԱ�ʵ��װ�ã�
��װ��A�в���������Բ����ƿ�����ܺͷ�Һ©�����Լ�WΪ����������Һ��
��װ��C�е��Լ�ΪNaCl��Һ��Ag2S����Һ�Ļ���B���Լ�ΪAg2S����Һ��
��ʵ�������C�г�����Ϊ���ɫ��B��û�����Ա仯��
���C�з�Ӧ�Ļ�ѧ����ʽ��
��Ag2S+��NaCl+��O2+��H2O?��AgCl+��S+��NaOH
C��NaCl�������ǣ�������Ag2S������Sʱ��Ag+������NaCl������������������ӽ������AgCl������ʹc��Ag+����С��������������ԭ��Ӧ��ƽ�����ƣ�
| �������� | ���� | ̼����� | ����� | ��ˮ |
| ��Һ��pH | 7 | 8 | 5 | 11 |
| A�� | ̼����� | B�� | ���� | C�� | ��ˮ | D�� | ����� |
| A�� | ���� | B�� | ��ˮ�Ҵ� | C�� | ʯ��ˮ | D�� | H2 |
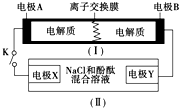 ��ͼΪһ�ֿɳ���ص�ʾ��ͼ�����е����ӽ���Ĥֻ����K+ͨ�����õ�طŵ硢���Ļ�ѧ��Ӧ����ʽΪ��2K2S2+KI3 $?_{���}^{�ŵ�}$K2S4+3KI��װ�ã���Ϊ���ص�ʾ��ͼ�����պϿ���Kʱ���缫X������Һ�ȱ�죮��պ�Kʱ������˵����ȷ���ǣ�������
��ͼΪһ�ֿɳ���ص�ʾ��ͼ�����е����ӽ���Ĥֻ����K+ͨ�����õ�طŵ硢���Ļ�ѧ��Ӧ����ʽΪ��2K2S2+KI3 $?_{���}^{�ŵ�}$K2S4+3KI��װ�ã���Ϊ���ص�ʾ��ͼ�����պϿ���Kʱ���缫X������Һ�ȱ�죮��պ�Kʱ������˵����ȷ���ǣ�������| A�� | K+������ͨ�����ӽ���Ĥ | |
| B�� | �缫A�Ϸ����ķ�ӦΪ��3I--2e-�TI3- | |
| C�� | �缫X�Ϸ����ķ�ӦΪ��2Cl--2e-�TCl2�� | |
| D�� | ����0.1 mol K+ͨ�����ӽ���Ĥ��X�缫�ϲ���1.12 L���� |
 Ԫ�ؼ��仯�����ѧϰ��Ӧ������ѧ��ѧ����Ҫ����֮һ��
Ԫ�ؼ��仯�����ѧϰ��Ӧ������ѧ��ѧ����Ҫ����֮һ��