��Ŀ����
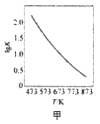
����Ŀ��KClO3��KHSO3�ܷ�����Ӧ��ClO3��+HSO3����SO42��+ Cl��+ H+(δ��ƽ)����֪�÷�Ӧ��������c(H+)��������ӿ졣��ͼΪ��ClO3���ڵ�λʱ�������ʵ���Ũ�ȱ仯��ʾ�ĸ÷�Ӧ��-tͼ������˵����ȷ��
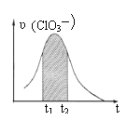
A. ��Ӧ��ʼʱ�������������c(H+)����
B. ������Ϊ��(Cl��)����-t������ͼ�����߲�����ȫ�غ�
C. �����������½�����Ҫԭ���Ƿ�Ӧ���淴Ӧ�������
D. ͼ����Ӱ����������Ա�ʾt1-t2ʱ����c(SO42-)������
���𰸡�A
��������
1molClO3-�μ�������ԭ��Ӧ�õ�6mol���ӣ�1mol������������Ӳμ�������ԭ��Ӧʧȥ2mol���ӣ����Ե�ʧ���ӵ���С��������6����ClO3-�ļ�������1��������������ӵļ�������3����ԭ�Ӹ����غ�ɵ÷�Ӧ����ʽΪClO3-+3HSO3-=3SO42-+Cl-+3H+��
A��������Ϣ��֪��Ӧ��������c��H+����������ӿ죬�ɷ���ʽ��֪����Ӧ��ʼʱ���ŷ�Ӧ�Ľ��У�c��H+����������Ӧ�����ʼӿ죬��A��ȷ��
B���ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ��ɷ���ʽ��֪����ClO3-��������Cl-��=1��1����������Ϊ����Cl-������-t������ͼ�������غϣ���B����
C����ŷ�Ӧ�Ľ��У���Ӧ���Ũ�ȼ��٣���Ӧ���ʼ�С�����Ժ��ڷ�Ӧ�����½�����Ҫԭ���Ƿ�Ӧ��Ũ�ȼ�С����C����
D�ͼ����Ӱ����������Ա�ʾt1-t2ʱ����c(ClO3-)���������ɷ���ʽ��֪��SO42-�Ļ�ѧ��������ClO3-��3������t1-t2ʱ����c(SO42-)������Ӧ��c(ClO3-)��������3������D����
��ѡA��

 ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д� ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�����Ŀ�����������ؾ���Kx[Fe(C2O4)y]��3H2O��һ�ֹ������ϣ�������һ���Ʊ����������ؾ����ʵ�����̡�
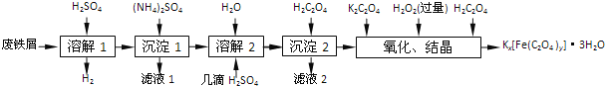
��֪��(NH4)2SO4��FeSO4��7H2O��Ī����[(NH4)2SO4��FeSO4��6H2O]���ܽ�������
�¶�/�� | 10 | 20 | 30 | 40 | 50 |
(NH4)2SO4/g | 73.0 | 75.4 | 78.0 | 81.0 | 84.5 |
FeSO4��7H2O/g | 40.0 | 48.0 | 60.0 | 73.3 | �D |
(NH4)2SO4��FeSO4��6H2O/g | 18.1 | 21.2 | 24.5 | 27.9 | 31.3 |
(1)����м�ڽ��С��ܽ�1��ǰ��������5% Na2CO3��Һ�м��������ӣ���ϴ�Ӹɾ���Na2CO3��Һ��������________��
(2)���ܽ�1��Ӧ��֤��м�Թ�������Ŀ����___________�����ܽ�2���ӡ�����H2SO4����������________��
(3)�����ֽ⡱�Ʊ�Ī���ξ���Ļ���ʵ�鲽���ǣ�����Ũ����________�����ˡ����Ҵ�ϴ�ӡ�������Ҵ�ϴ�ӵ�Ŀ����____________��
(4)��������ʱ�õ���FeC2O4��2H2O��������ˮϴ�Ӹɾ�����������Ƿ�ϴ�Ӹɾ��ķ�����_______��
(5)���ᾧ��Ӧ����Һ���ںڰ����ȴ����������������������ԭ����__________��
(6)�벹ȫ�ⶨ���������ز�Ʒ��Fe3��������ʵ�鲽��[��ѡ�Լ���KMnO4��Һ��п�ۡ����ۡ�NaOH��Һ��
����1��ȷ��ȡ���Ʊ��IJ��������ؾ���a g�����250 mL����Һ��
����2������Һ����ȡ25.00 mL����Һ����ƿ�У�����ϡH2SO4�ữ��_________��C2O42-ת��ΪCO2����ȥ��
����3������2������Һ��______________��
����4����c mol��L��1 KMnO4����Һ�ζ�����3������Һ���յ㣬����V mL KMnO4����Һ��