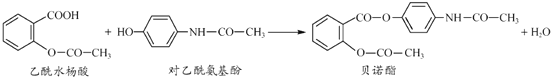
��Ŀ����
����Ŀ��ij��ѧ��ȤС��ͬѧչ����Ư����������(NaClO2)���о���
ʵ�����ȡNaClO2����
��֪��NaClO2������Һ���¶ȵ���38 ��ʱ�����ľ�����NaClO2��3H2O������38 ��ʱ�����ľ�����NaClO2������60 ��ʱNaClO2�ֽ��NaClO3��NaCl��Ba(ClO)2������ˮ��
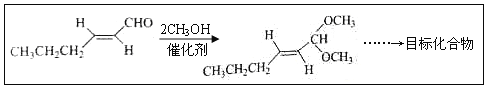
������ͼ��ʾװ�ý���ʵ�顣

��1��װ�â۵�������______________________________��
��2��װ�â��в���ClO2�Ļ�ѧ����ʽΪ________________________________��
��3����װ�âܷ�Ӧ�����Һ���NaClO2����IJ�������Ϊ���ټ�ѹ��55 �������ᾧ���ڳ��ȹ��ˣ���______________���ܵ���60 �����õ���Ʒ��
��4��װ�â١��ݵ�������______________________________��
��5�����ʵ���������NaClO2�����Ƿ�������Na2SO4������������ȡ����������������ˮ��___________________________________��
ʵ��ⶨij����������Ʒ�Ĵ��ȡ�
�������ʵ�鷽����������ʵ�飺
��ȷ��ȡ��������������Ʒm g���ձ��У�������������ˮ�����ĵ⻯�ؾ��壬�ٵ���������ϡ���ᣬ��ַ�Ӧ(��֪��ClO2-��4I����4H��===2H2O��2I2��Cl��)�������û��Һ���100 mL������Һ��
����ȡ25.00 mL������Һ����ƿ�У���c mol��L��1 Na2S2O3��Һ�ζ������ζ��յ㡣�ظ�2�Σ�������ı���Һ�������ƽ��ֵΪV mL(��֪��I2��2S2O32-===2I����S4O62-)��
��6���ζ���ʹ�õ�ָʾ����______________���ﵽ�ζ��յ�ʱ������Ϊ__________��
��7����Ʒ��NaClO2����������Ϊ______________(�ú�m��c��V�Ĵ���ʽ��ʾ)��
���𰸡���ֹ���� 2NaClO3��Na2SO3��H2SO4(Ũ)==2ClO2����2Na2SO4��H2O ��38��60 �����ˮϴ�� ���ն����ClO2���壬��ֹ��Ⱦ �μӼ���BaCl2��Һ�����а�ɫ�������֣�����Na2SO4�����ް�ɫ�������֣���Na2SO4 ������Һ ��Һ����ɫ��Ϊ��ɫ�Ұ���Ӳ���ɫ ![]() % ��
% ��![]()
��������
���⿼�����̽�����ʵ���ɻ�������ʵĺ���, �Ʊ�ʵ�鷽������ơ�
��1��װ�â������巴Ӧ��װ����ѹǿ���ͣ�װ�â۷�ֹ������
��2���������ƾ��л�ԭ�ԣ��ڷ�Ӧ������ԭ����װ�âܷ�Ӧ�����Һ���NaClO2���壬��װ�â�������NaClO2��ClԪ�صĻ��ϼ۽��ͣ�˫��ˮӦ���ֻ�ԭ�ԣ����������ɣ����ԭ���غ��֪������ˮ���ɣ���ƽ��д����ʽ��
��3������Һ����ȡ���壬һ����������ᾧ�����ˡ�ϴ�ӡ�����ķ�����ע���¶ȿ��ƣ�
��4��װ�â١��ݵ����������ն����ClO2���壬��ֹ��Ⱦ��
��5����������Ϣ��֪ Ba(ClO)2������ˮ����������NaClO2�����Ƿ�������Na2SO4���ɵμӼ���BaCl2��Һ�����а�ɫ�������֣�����Na2SO4�����ް�ɫ�������֣���Na2SO4��
��6����Ӧ����ʱ���ⷴӦ��ȫ���μ����һ��Һ��ʱ��Һ����ɫ��Ϊ��ɫ�Ұ�����ڲ���ɫ��˵������ζ��յ㣻
��7�����ݹ�ϵʽNaClO2��2I2��4S2O32-���м��㡣�ݴ˽��
(1)װ�â��ǰ�ȫƿ�ܷ�ֹ��������С���Ϊ����ֹ������
(2)�������ƾ��л�ԭ�ԣ��ڷ�Ӧ������ԭ����װ�â��в���ClO2�ķ�Ӧ����������������Һ��������������Ϊ�����ƣ���������ԭΪ�������ȣ���Ӧ�Ļ�ѧ����ʽΪ��2NaClO3+Na2SO3+H2SO4=2ClO2��+2Na2SO4+H2O����С���Ϊ��2NaClO3+Na2SO3+H2SO4=2ClO2��+2Na2SO4+H2O��
(3)����Һ����ȡ���壬һ����������ᾧ�����ˡ�ϴ�ӡ�����ķ�����Ϊ��ֹ��������NaClO23H2O��Ӧ���ȹ��ˣ�����Ŀ��Ϣ��֪��Ӧ�����¶�38��60�����ϴ�ӣ�����60������С���Ϊ����38��60 �����ˮϴ�ӡ�
(4)װ�â١��ݵ����������ն����ClO2���壬��ֹ��Ⱦ����С���Ϊ�����ն����ClO2���壬��ֹ��Ⱦ��
(5)��������Ϣ��֪ Ba(ClO)2������ˮ����������NaClO2�����Ƿ�������Na2SO4���ɵμӼ���BaCl2��Һ�����а�ɫ�������֣�����Na2SO4�����ް�ɫ�������֣���Na2SO4����С��Ϊ�� �μӼ���BaCl2��Һ�����а�ɫ�������֣�����Na2SO4�����ް�ɫ�������֣���Na2SO4��
(6)�������۱���ɫ����Ӧ����ʱ���ⷴӦ��ȫ���μ����һ��Na2S2O3��Һʱ��Һ����ɫ��Ϊ��ɫ�Ұ�����ڲ���ɫ��˵������ζ��յ㡣��С���Ϊ��������Һ����Һ����ɫ��Ϊ��ɫ�Ұ���Ӳ���ɫ��
(7) ����Ʒ��NaClO2����������Ϊa����
NaClO22I24S2O32
90.5g 4mol
magcmolL1��V��103L��100ml/25ml��
����90.5g/mag=4mol/(cmolL1��V��103L��100ml/25ml)��
���a= ![]() %����С���Ϊ��
%����С���Ϊ��![]() % ��
% ��![]() ��
��

 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�