��Ŀ����
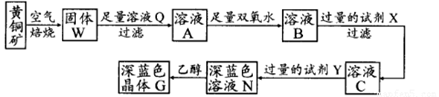
(12��) ��ұ���ķ���������Ϊԭ����ȡ��ϸ��-���������Ƚ��ͻ�����Ⱦ�ֿ��������Դ�������ʡ���֪���ҵ���Ҫ�ɷ�ΪAl2O3������������SiO2��FeO��Fe2O3�������Ʊ�ʵ���������£�
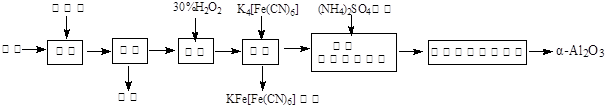
��1��ͼ�С���������ѧ����ʽΪ ��
��2��ͼ�С����ˡ�����Һ�н��������ӳ��˺��е�Al3���������� (�ѧʽ)��
��3����30%��H2O2��Һ���������ӷ�Ӧ����ʽΪ ��
��4������������茶��壬��������Ҫ��ӦΪ��
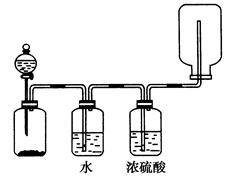
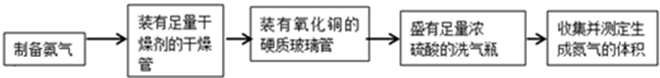
4[NH4Al(SO4)2��12H2O]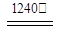 2Al2O3 + 2NH3��+ N2��+ 5SO3��+ 3SO2��+ 53H2O,������������ͨ��ͼ9��ʾ��װ�á�
2Al2O3 + 2NH3��+ N2��+ 5SO3��+ 3SO2��+ 53H2O,������������ͨ��ͼ9��ʾ��װ�á�


�ټ���ƿ���ռ����������� (�ѧʽ)��
����������NaHSO3��Һ���յ����ʳ���H2O��g����� (�ѧʽ)��
��KMnO4��Һ��ɫ��MnO4����ԭΪMn2+�������������ӷ�Ӧ����ʽΪ ��
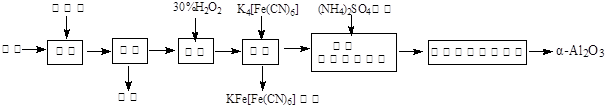
��1��ͼ�С���������ѧ����ʽΪ ��
��2��ͼ�С����ˡ�����Һ�н��������ӳ��˺��е�Al3���������� (�ѧʽ)��
��3����30%��H2O2��Һ���������ӷ�Ӧ����ʽΪ ��
��4������������茶��壬��������Ҫ��ӦΪ��
4[NH4Al(SO4)2��12H2O]
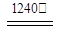 2Al2O3 + 2NH3��+ N2��+ 5SO3��+ 3SO2��+ 53H2O,������������ͨ��ͼ9��ʾ��װ�á�
2Al2O3 + 2NH3��+ N2��+ 5SO3��+ 3SO2��+ 53H2O,������������ͨ��ͼ9��ʾ��װ�á�

�ټ���ƿ���ռ����������� (�ѧʽ)��
����������NaHSO3��Һ���յ����ʳ���H2O��g����� (�ѧʽ)��
��KMnO4��Һ��ɫ��MnO4����ԭΪMn2+�������������ӷ�Ӧ����ʽΪ ��
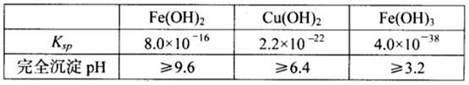
��1��2K4[Fe(CN)6]+ Fe 2(SO4)3 = 2KFe[Fe(CN)6]��+3K2SO4��2�֣�
��2��Fe2+ Fe3+��2�֣�
��3��2Fe2++H2O2+2H+ = 2Fe3++2H2O��2�֣�
��4����N2��2�֣���SO3��NH3��2�֣�ȱ©�����֣���
��2MnO4�� +5SO2 + 2H2O = 2Mn2+ + 5SO42��+4H+��2�֣�
��2��Fe2+ Fe3+��2�֣�
��3��2Fe2++H2O2+2H+ = 2Fe3++2H2O��2�֣�
��4����N2��2�֣���SO3��NH3��2�֣�ȱ©�����֣���
��2MnO4�� +5SO2 + 2H2O = 2Mn2+ + 5SO42��+4H+��2�֣�
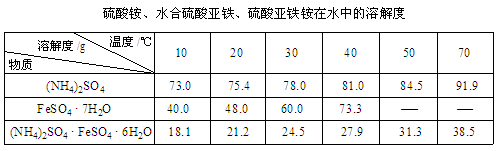
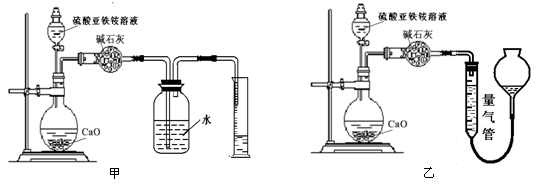
�����������1����K4[Fe(CN)6]����KFe[Fe(CN)6]����2������������������������ᣬ�������費���γ���������3����Һ�е��������Ӳ��׳�ȥ�����������Ϊ�������ٳ�����ȥ����4����ͨ������������������Һ��ȥ��������������ͨ�����������Һ��ȥ�����������壬���ռ�������Ϊ��������SO3��NH3���ܽ���ˮ��ˮ��Ӧ����KMnO4��Һ���������Ӧ��������������Ϊ��������ӡ�

��ϰ��ϵ�д�
 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д� ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
�����Ŀ




 ������ƿ�У���0.05mol/L������Һ���еζ������ı���Һ20.00ml����ش��������⣺
������ƿ�У���0.05mol/L������Һ���еζ������ı���Һ20.00ml����ش��������⣺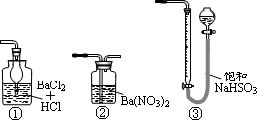
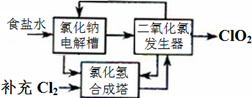
 2ClO2��+2Na2SO4��H2O
2ClO2��+2Na2SO4��H2O H++OH-�� ________________�������ӷ���ʽ��ʾ��.
H++OH-�� ________________�������ӷ���ʽ��ʾ��.