��Ŀ����
(15��)����������ֳ�Ī���Σ���dz��ɫ���塣���ڿ����б�һ���������ȶ����dz��õ�Fe2+�Լ���ijʵ��С�����ù�ҵ����м��ȡĪ���Σ����ⶨ�䴿�ȡ�
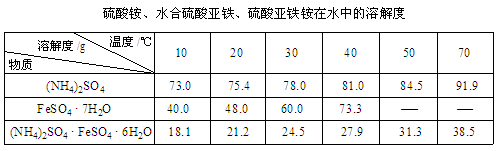
��֪:��
��Ī�������Ҵ��ܼ������ܡ�
��Ī���ε���ȡ

�Է�����
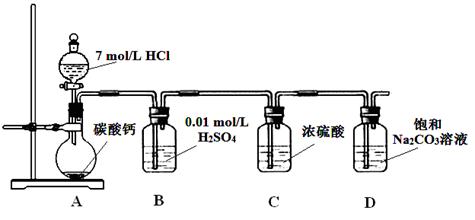
��1������2�м��ȷ�ʽ ���ֱ�Ӽ��ȡ��p��ˮԡ���ȡ���ɳԡ��������������м����ʣ��ʱ�������ȹ��ˣ���ԭ���� ��
��2������3�а�����ʵ��������� ��
��3����ƷĪ��������� ϴ�ӣ�����ĸ��ţ���
a������ˮ b���Ҵ� c����Һ
��Ϊ�ⶨ���������(NH4)2SO4?FeSO4?6H2O���崿�ȣ�ijѧ��ȡm g�����������Ʒ���Ƴ�500 mL��Һ������������ɣ��ס��ҡ�����λͬѧ�������������ʵ�鷽������ش�
(��)����һ��ȡ20.00 mL�����������Һ��0.1000 mol��L��1������KMnO4��Һ�����ν��еζ���
(��)��������ȡ20.00 mL�����������Һ��������ʵ�顣

��1����ʵ���������ȷ��������һ�IJⶨ�������С�ڷ������������ԭ��Ϊ
����֤�Ʋ�ķ���Ϊ�� ��
(��)��������(ͨ��NH4+�ⶨ)ʵ�����ͼ������ʾ��ȡ20.00 mL�����������Һ���и�ʵ�顣
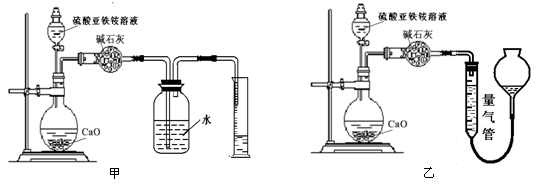
��2����װ�� ����ס����ҡ�����Ϊ�������ж������� ��������������Լ��� ������ĸ��š���ѡ���ҡ�����˿գ���ѡ���ס��˿տɲ����
a��ˮ b������NaHCO3��Һ c��CCl4
�������NH3�����ΪV L(������Ϊ��״����)�������������茶���Ĵ���Ϊ ��
��֪:��

��Ī�������Ҵ��ܼ������ܡ�
��Ī���ε���ȡ

�Է�����
��1������2�м��ȷ�ʽ ���ֱ�Ӽ��ȡ��p��ˮԡ���ȡ���ɳԡ��������������м����ʣ��ʱ�������ȹ��ˣ���ԭ���� ��
��2������3�а�����ʵ��������� ��
��3����ƷĪ��������� ϴ�ӣ�����ĸ��ţ���
a������ˮ b���Ҵ� c����Һ
��Ϊ�ⶨ���������(NH4)2SO4?FeSO4?6H2O���崿�ȣ�ijѧ��ȡm g�����������Ʒ���Ƴ�500 mL��Һ������������ɣ��ס��ҡ�����λͬѧ�������������ʵ�鷽������ش�
(��)����һ��ȡ20.00 mL�����������Һ��0.1000 mol��L��1������KMnO4��Һ�����ν��еζ���
(��)��������ȡ20.00 mL�����������Һ��������ʵ�顣

��1����ʵ���������ȷ��������һ�IJⶨ�������С�ڷ������������ԭ��Ϊ
����֤�Ʋ�ķ���Ϊ�� ��
(��)��������(ͨ��NH4+�ⶨ)ʵ�����ͼ������ʾ��ȡ20.00 mL�����������Һ���и�ʵ�顣

��2����װ�� ����ס����ҡ�����Ϊ�������ж������� ��������������Լ��� ������ĸ��š���ѡ���ҡ�����˿գ���ѡ���ס��˿տɲ����
a��ˮ b������NaHCO3��Һ c��CCl4
�������NH3�����ΪV L(������Ϊ��״����)�������������茶���Ĵ���Ϊ ��
��15�֣�
I����1��ˮԡ����(1�� )
��ֹFe2+��������ͬʱ�ȹ��˿ɷ�ֹ���������Ծ�����ʽ������2�֣�
��2������������Ũ���ᾧ��2�֣�
��3�� b��1�� ��
II����1�� Fe2+�ѱ������������� (2��)
ȡ���������������Һ����������KSCN��Һ������Һ��ΪѪ��ɫ��˵��Fe2+��
�������������� (2��)
��2�����ң�1�֣� ��װ�û���ֵ���(1��) c ��1�֣�
�� (2��)
(2��)
I����1��ˮԡ����(1�� )
��ֹFe2+��������ͬʱ�ȹ��˿ɷ�ֹ���������Ծ�����ʽ������2�֣�
��2������������Ũ���ᾧ��2�֣�
��3�� b��1�� ��
II����1�� Fe2+�ѱ������������� (2��)
ȡ���������������Һ����������KSCN��Һ������Һ��ΪѪ��ɫ��˵��Fe2+��
�������������� (2��)
��2�����ң�1�֣� ��װ�û���ֵ���(1��) c ��1�֣�
��
 (2��)
(2��)���������I.��1������2�е��¶ȿ�����70-75�棬����ѡ��ˮԡ���ȣ�Fe2+�ױ����������������¶Ƚ��������������ܽ�ȼ�С�����Ա�������м����ʣ��ʱ�������ȹ��ˣ���ԭ���Ƿ�ֹFe2+�ױ�������ͬʱ�ȹ��˿ɷ�ֹ���������Ծ�����ʽ������
��2���������������Һ�����壬�м���Ҫ��������������Ũ���ᾧ��
��3����ΪĪ�������Ҵ��ܼ������ܣ�����ѡ�����Ҵ�ϴ�ӣ���ѡb��
II.��1�������Ը��������Һ�ζ���ԭ�����������ӱ����Ը��������Һ����������������Һ����������������ӵ������Ӷ����㾧��Ĵ��ȣ����Ȼ�����Һ�ζ���ԭ��������������뱵���ӷ�Ӧ�������ᱵ���������ó���������������������ӵ������Ӷ����㾧��Ĵ��ȡ���������Ӳ��ᷢ���仯�����Է���һ�IJⶨ�������С�ڷ������������ԭ��ΪFe2+�ѱ�����������������֤�Ʋ�ķ���������֤��Һ���Ƿ���������ӣ����������ȡ���������������Һ����������KSCN��Һ������Һ��ΪѪ��ɫ��˵��Fe2+�ѱ���������������
��2����װ���ұȽϺ�������Ϊ��װ�û���ֵ��������������������������ѡ����װ�ã�����������ʢ�ŵ��Լ������Ȼ�̼����Ϊ����������ˮ�����������Ȼ�̼���������ݱȽ�ȷ�����Դ�ѡc��
�ڸ�����������淋Ļ�ѧʽ�ó�2NH3��(NH4)2SO4?FeSO4?6H2O������500mL��Һ����������茶��������ΪVL/22.4L/mol/2��392g/mol��25���䴿��ΪVL/22.4L/mol/2��392g/mol��25/mg��100%=
 ��
��
��ϰ��ϵ�д�
�����Ŀ
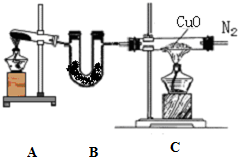

 2Al2O3 + 2NH3��+ N2��+ 5SO3��+ 3SO2��+ 53H2O,������������ͨ��ͼ9��ʾ��װ�á�
2Al2O3 + 2NH3��+ N2��+ 5SO3��+ 3SO2��+ 53H2O,������������ͨ��ͼ9��ʾ��װ�á�


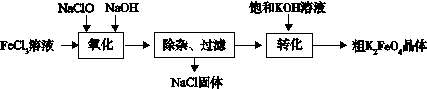
 3Zn(OH)2 + 2Fe(OH)3 + 4KOH
3Zn(OH)2 + 2Fe(OH)3 + 4KOH