��Ŀ����
16��X��Y��Z��WΪǰ������Ԫ���γɵ��������ǵĵ���������ȣ���֪XΪ������˫ԭ�������ӣ�YΪ˫ԭ�ӷ��ӣ�Z����ΪҺ�壬��ˮ��Һ�������ԣ�W������ˮ����1��X����������ɵ����ӻ����ﳣ���¸�ˮ��Ӧ����һ�ֿ���ȼ�����壬�÷�Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2H2O�T4NaOH+O2����
��2����YΪ��ˮ�;��ҷ�Ӧ�����ʣ���Y�ĵ���ʽΪ
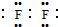 ����YΪ�������ʯī�缫���Y��ˮ��Һ�����ⷴӦΪ2HCl$\frac{\underline{\;���\;}}{\;}$H2��+C12����
����YΪ�������ʯī�缫���Y��ˮ��Һ�����ⷴӦΪ2HCl$\frac{\underline{\;���\;}}{\;}$H2��+C12������3������ϡ�����м���ͭ�ۺ��ټ�z������ͭ�����ܽ⣬�÷�Ӧ�����ӷ���ʽΪ��Cu+H2O2+2H�TCu2++2H2O��
��4��w������ǡ����ȫ��Ӧ�õ��������ʣ������������⻯������о���10�����ӣ���һ����������������C3H4�����ĵ�������ȣ���W�Ļ�ѧʽΪCH3F��
���� X��Y��Z��WΪǰ������Ԫ���γɵ��������ǵĵ���������ȣ�XΪ������˫ԭ�������ӣ�����������ɵ����ӻ����ﳣ���¸�ˮ��Ӧ����һ�ֿ���ȼ�����壬Ӧ���ǹ���������ˮ��Ӧ����������������������XΪO22-����������18�����ӣ�YΪ˫ԭ�ӷ��ӣ���YΪF2��HCl��Z����ΪҺ�壬��ˮ��Һ�������ԣ���ZΪH2O2��W������ˮ��������ǡ����ȫ��Ӧ�õ��������ʣ������������⻯������о���10�����ӣ������⻯��ֻ��ΪH2O��HF��WΪ������������֪WΪCH3F�����ɵ�������CO2��C3H4�����ĵ�������ȣ���Ϊ22���������⣮
��� �⣺X��Y��Z��WΪǰ������Ԫ���γɵ��������ǵĵ���������ȣ�XΪ������˫ԭ�������ӣ�����������ɵ����ӻ����ﳣ���¸�ˮ��Ӧ����һ�ֿ���ȼ�����壬Ӧ���ǹ���������ˮ��Ӧ����������������������XΪO22-����������18�����ӣ�YΪ˫ԭ�ӷ��ӣ���YΪF2��HCl��Z����ΪҺ�壬��ˮ��Һ�������ԣ���ZΪH2O2��W������ˮ��������ǡ����ȫ��Ӧ�õ��������ʣ������������⻯������о���10�����ӣ������⻯��ֻ��ΪH2O��HF��WΪ������������֪WΪCH3F�����ɵ�������CO2��C3H4�����ĵ�������ȣ���Ϊ22���������⣮
��1������������ˮ��Ӧ����������������������Ӧ����ʽΪ��2Na2O2+2H2O�T4NaOH+O2�����ʴ�Ϊ��2Na2O2+2H2O�T4NaOH+O2����
��2����YΪ��ˮ�;��ҷ�Ӧ�����ʣ���YΪF2������ʽΪ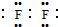 ����YΪ�������ΪHCl����ʯī�缫���HCl��ˮ��Һ�����ⷴӦΪ��2HCl$\frac{\underline{\;���\;}}{\;}$H2��+C12�����ʴ�Ϊ��
����YΪ�������ΪHCl����ʯī�缫���HCl��ˮ��Һ�����ⷴӦΪ��2HCl$\frac{\underline{\;���\;}}{\;}$H2��+C12�����ʴ�Ϊ��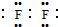 ��2HCl$\frac{\underline{\;���\;}}{\;}$H2��+C12����
��2HCl$\frac{\underline{\;���\;}}{\;}$H2��+C12����
��3������ϡ�����м���ͭ�ۺ��ټ�H2O2������ͭ�����ܽ⣬�÷�Ӧ�����ӷ���ʽΪ��Cu+H2O2+2H�TCu2++2H2O���ʴ�Ϊ��Cu+H2O2+2H�TCu2++2H2O��
��4��������������֪��W�Ļ�ѧʽΪCH3F���ʴ�Ϊ��CH3F��
���� ���⿼�������ƶϣ��ؼ��Ǹ�����X���������γɵĻ�������ˮ�ķ�Ӧ�ж�������18���ӣ���Ҫѧ���������ճ���10���ӡ�18 ���������Ѷ��еȣ�

 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д���1��ѡȡ��Ҫ��ʵ��װ�ã���ȷ������˳��ΪCAB������ţ���
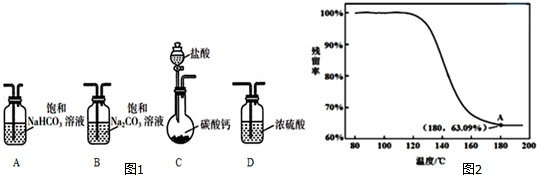
��2��Ϊȷ���ƵõĹ�����Ʒ�Ǵ�����NaHCO3��С��ͬѧ�������ʵ�鷽����
����������Ʒ��Һ�뱥�ͳ���ʯ��ˮ��Ӧ���۲�����
�ҷ���������Ʒ��Һ��BaCl2��Һ��Ӧ���۲�����
���������ⶨpH����
�����������ط�������
����������������������������
��Ϊ�ж��ҷ����Ŀ����ԣ�ijͬѧ�ô�����NaHCO3���Ƶ���Һ����BaCl2��Һ�������Ͻ���ʵ�飬������£�
NaHCO3��Һ BaCl2Ũ�� | 0.2mol•L-1 | 0.1mol•L-1 | 0.02mol•L-1 |
| 0.2mol•L-1 | ���� | ���� | �������� |
| 0.1mol•L-1 | ���� | �������� | ������ |
| 0.02mol•L-1 | �������� | ������ | ������ |
[��֪��0��l mol•L-1 NaHCO3��Һ�������c��CO32-��Ϊ0.001l mol•L-1��Ksp��BaCO3��=5.1��10-9]
��Qc=c��Ba2+����c��CO32-��=$\frac{0.2}{2}$��0.0011=1.1��10-4��5.1��10-9��
��ii���������ǣ�������������������ӷ���ʽBa2++2HCO3-=BaCO3��+CO2��+H2O��
����pH�Ʋⶨ�ı��������£�
ȡm�˵Ĺ��������ܽ���ˮ���V mL����Һ����pH�Ʋ�pH��
��Ӧ�����ʵ���ǣ���ȡ�������ķ�����NaHC03����ˮ�����V mL����Һ����pH�Ʋ�pH
�ܽ��ж�����ʵ�飬�õ�������������¶ȱ仯��������ͼ2��ʾ������A������õ��Ľ������ƵõĹ�����Ʒ�Ǵ�����NaHCO3��
��������=$\frac{ʣ����������}{ԭʼ���������}$��100%��
| A�� | C1��C2 | B�� | C1=C2 | C�� | C1��C2 | D�� | ��ȷ�� |
| A�� | ����£�22.4L SO3����3NA��Oԭ�� | |
| B�� | ���³�ѹ�£�27g Al������NaOH ��Һ��Ӧ����1.5NA��H2 | |
| C�� | ���³�ѹ�£�32g�����������O3 ���Ļ�����к���NA����ԭ�� | |
| D�� | ���ʵ���Ũ��Ϊ1 mol•L-1 �� K2SO4 ��Һ�У���2NA��K+ |
| A�� | 2�� | B�� | 4�� | C�� | 3�� | D�� | 6�� |


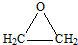 ��
��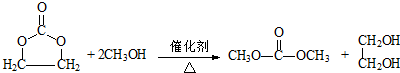 ��
��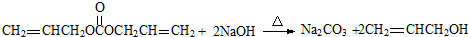 ��
�� ��
�� ����C��D�γɵĻ�����ĵ���ʽ
����C��D�γɵĻ�����ĵ���ʽ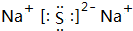 ��
��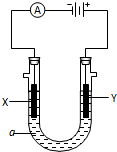 ij��ѧʵ��С����Ҫ2mol•L-1��NaCl��Һ98ml������NaCl���������ƣ���ش��������⣺
ij��ѧʵ��С����Ҫ2mol•L-1��NaCl��Һ98ml������NaCl���������ƣ���ش��������⣺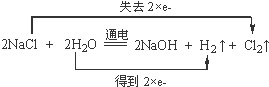 ��
��