��Ŀ����
20��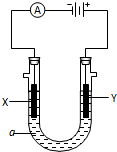 ij��ѧʵ��С����Ҫ2mol•L-1��NaCl��Һ98ml������NaCl���������ƣ���ش��������⣺
ij��ѧʵ��С����Ҫ2mol•L-1��NaCl��Һ98ml������NaCl���������ƣ���ش��������⣺��1�����ȡNaCl11.7g
��2�����ƹ����У�����Ҫ������������ţ�BG��
A��ҩ�� B���ƾ��� C��������ƽ D���ձ� E�������� F��ϴƿ G��������
���ʵ�黹ȱ�ٵ�������100mL����ƿ����ͷ�ιܣ�
��3�������ƹ����У����в���������������ҺŨ��ƫ�͵��Т٢ڢݣ�����ţ���
�ٳ���NaCl����ʱ��������NaCl����λ�÷ŷ���1g���������룩
������ȡ��NaCl���庬���������� ��ת��ǰ������ƿ�к�����������ˮ
�ܶ���ʱ�����ӿ̶��� ��ת��ʱ������Һ�彦��
��4����ʵ��С�����������Ƶ���Һ��������ͼ��ʾ�ĵ��ʵ�飮
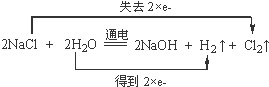
��д���õ�ⷴӦ�Ļ�ѧ����ʽ�����ڻ�ѧ����ʽ�ϱ������ת�Ƶķ������Ŀ��
 ��
���ڵ��ʱ����Y�������������ɵ�����ͨ��ʢ��NaI��Һ���Թ��У��ټ������Ȼ�̼��������۲쵽��������C��
A����Һ�ֲ㣬�ϲ���ɫ���²�Ⱥ�ɫ
B����Һ�ֲ㣬�ϲ�Ⱥ�ɫ���²���
C����Һ�ֲ㣬�ϲ���ɫ���²��Ϻ�ɫ
D����Һ�ֲ㣬�ϲ��Ϻ�ɫ���²���
��д����ҵ��Y�����ɵ���������ȡƯ�۵Ļ�ѧ����ʽ��2Cl2+2Ca��OH��2�TCa��ClO��2+CaCl2+2H2O��
���� ��1������ʵ������98mL������ƿ��ֻ��ѡ��100mL����ƿ���Ƴ�100mL��Һ�����㣻
��2���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��3������c=$\frac{n}{V}$��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��4���ٵ�ⱥ��ʳ��ˮ�ķ���ʽΪ2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+Cl2��+H2������Ӧ��ClԪ�صĻ��ϼ����ߣ�HԪ�صĻ��ϼ۽��ͣ�ÿת��2mol��������1���������ݻ�ѧ����ʽ���㣮
����������Cl-�ŵ�����Cl2��ͨ��ʢ��NaI��Һ���Թ����ܽ�I2�û������ټ������Ȼ�̼��������ܽ��ⵥ����ȡ�����������Ȼ�̼���ܶȱ�ˮ������������
�۹�ҵ��Ư������������ʯ���鷴Ӧ����ȡ��
��� �⣺��1��ʵ������98mL������ƿ��ֻ��ѡ��100mL����ƿ���Ƴ�100mL��Һ���������NaCl������m=nM=CVM=2mol/L��0.1L��58.5g/mol=11.7g���ʴ�Ϊ��11.7g��
��2���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪ʵ���������Ҫ������ƽ��ҩ�ס��ձ�����������100mL����ƿ����ͷ�ιܡ�ϴƿ������������ҪBG����ȱ��100mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��BG��100mL����ƿ����ͷ�ιܣ�
��3���ٳ���NaCl����ʱ��������NaCl����λ�÷ŷ���1g���������룩���ᵼ��ҩƷ������ƫС����������Һ��Ũ��ƫ�ͣ�
������ȡ��NaCl���庬���������ʣ����Ȼ��Ƶ�ʵ������ƫС�������Ƴ�����Һ��Ũ��ƫ�ͣ�
��������ƿδ���T����������Һ������ҺŨ����Ӱ�죬��ΪֻҪ����ʱ��ȷ������ˮ��ԭ�����еĻ��Ǻ�������ģ���Ũ����Ӱ�죻
�ܶ���ʱ�����ӿ̶��ߣ��ᵼ����Һ���ƫС����Ũ��ƫ�ߣ�
��ת��ʱ������Һ�彦�����������ʵ���ʧ����������Һ��Ũ��ƫ�ͣ�
��ѡ�٢ڢݣ�
��4���ٵ�ⱥ��ʳ��ˮ�ķ���ʽΪ2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+Cl2��+H2������Ӧ��ClԪ�صĻ��ϼ�����2�ۣ�HԪ�صĻ��ϼ۽���2�ۣ���Ӧ��ת��2�����ӣ�����ת�Ƶķ������ĿΪ�� ��
��
�ʴ�Ϊ�� ��
��
����������Cl-�ŵ�����Cl2��ͨ��ʢ��NaI��Һ���Թ����ܽ�I2�û������ټ������Ȼ�̼��������ܽ��ⵥ����ȡ�����������Ȼ�̼������ˮ���ܶȱ�ˮ����Һ�ֲ㣬������Ȼ�̼��Һ���²㣬���Ϻ�ɫ��ˮ���ϲ㣬����ɫ����ѡC��
�۹�ҵ��Ư������������ʯ���鷴Ӧ����ȡ�ģ���ѧ����ʽΪ��2Cl2+2Ca��OH��2�TCa��ClO��2+CaCl2+2H2O���ʴ�Ϊ��2Cl2+2Ca��OH��2�TCa��ClO��2+CaCl2+2H2O��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ�������������������˵绯ѧ���й�֪ʶ���ۺ��Խ�ǿ�����ѶȲ���

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�| A�� | Cl-�Ľṹʾ��ͼ�� | B�� | ������Ľṹʽ��H-Cl-O | ||
| C�� | �������Ƶĵ���ʽ�� | D�� | ��Ԫ�ز���������Ԫ�� |
| A�� | �������B��A=C | |
| B�� | A��B�γɵĻ�������ֻ�������Ӽ���A��C�γɵĻ�������ֻ���й��ۼ� | |
| C�� | A��B��C����Ԫ����������ֲ�ͬ�Ļ����� | |
| D�� | A��C�γɵ�һ�ֻ�����������������л�ԭ�� |
| A�� | ��ϡ�����У�Ba2+��CO32-��Zn2+ | B�� | ������������Һ�У�H+��Mg2+��Cl- | ||
| C�� | ���������Һ�У�Na+��K+��OH- | D�� | ���Ȼ�����Һ�У�K+��Ca2+��N03- |
| A�� | ��ʹ����ʯ��ˮ����ǵ�����һ����CO2 | |
| B�� | ��ʹƷ����Һ��ɫ����������ֱ�����ɫ����ΪSO2 | |
| C�� | ��ŨH2SO4��Ӧ�ɲ���SO2��һ��������������� | |
| D�� | ij������Һ������������S2-��SO42-��MnO4- |
| A�� | HCl | B�� | Na2O2 | C�� | H2O | D�� | CaCl2 |
| A�� | ��Ȼֲ���ͳ�����һ���Һ̬��������ˮ���к㶨���۵㡢�е� | |
| B�� | ��ѿ�������ǵ�ˮ�������������ǣ��ʶ��߾�Ϊ��ԭ�Ͷ��� | |
| C�� | ����ʽΪC5H12��������һ�ȴ�����3�� | |
| D�� | ����ϩ����������ԭ�ӿ�����ͬһƽ���� |
| A�� | ��ع���ʱ������������Һ�ļ�����ǿ | |
| B�� | �����ĵ缫��Ӧʽ�ǣ�O2+4H++4e-=2H2O | |
| C�� | �����ĵ缫��Ӧʽ�ǣ�N2H4+4OH--4e-=4H2O+N2�� | |
| D�� | ��Һ���������������ƶ� |
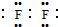 ����YΪ�������ʯī�缫���Y��ˮ��Һ�����ⷴӦΪ2HCl$\frac{\underline{\;���\;}}{\;}$H2��+C12����
����YΪ�������ʯī�缫���Y��ˮ��Һ�����ⷴӦΪ2HCl$\frac{\underline{\;���\;}}{\;}$H2��+C12����