��Ŀ����
����Ŀ��CO��H2����Ϊ�ϳ������úϳ������Ժϳ����ᡣ�ش��������⣺
(1)��֪CO��H2��CH3COOH��ȼ������H�ֱ�Ϊ-283.0kJ/mol��-285.8kJ/mol��-1255.0kJ/mol�����úϳ����ϳ�CH3COOH(l)�Ŀ�����̵��Ȼ�ѧ��Ӧ����ʽΪ___________________________________________��
(2)���ܱ������з����ϳ�����ķ�Ӧ�����п������CH3COOH���ʵĴ�ʩ��________��
A�����º��ݣ�ͨ���������� B.��ʱ�������������
C�������Ч���� D��ѹ���������
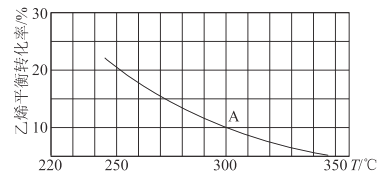
(3)��150��ʱ��2L���ܱ������з�����Ӧ��
2H2(g)+2CO(g)![]() CH3COOH(g) ��H>0����ʼͨ��4molH2��4molCO��CH3COOH������Ũ��������ʱ��仯���±���ʾ��
CH3COOH(g) ��H>0����ʼͨ��4molH2��4molCO��CH3COOH������Ũ��������ʱ��仯���±���ʾ��
ʱ��/min | 0 | 2 | 4 | 6 | 8 |
c(CH3COOH)/mol/L | 0 | 0.3 | 0.5 | 0.6 | 0.6 |
��0~2min����CO��ʾ�÷�Ӧ������Ϊ_________________�����ŷ�Ӧ�Ľ������������Ŀ���ԭ����____________________________________________________��
��150��ʱ�÷�Ӧ��ƽ�ⳣ������ֵԼΪ_________��������������һλС����
��ƽ�����ͨ��1molH2����CH3COOH(g)���������________�����ٴ�ͨ��1molH2��1molCO����CH3COOH(g)���������_________�����������С�����ߡ����䡱��
(4)һ���¶��£��ݻ���Ϊ1L�������ܱ�����֮�н�������ʵ�飺
ʵ���� | ��ʼͶ�� |
�� | 2molH2��2molCO |
�� | 1molCH3COOH(g) |
��ʵ�����H2��ʵ�����CH3COOH��ת������ʱ��仯ͼʾ���£�
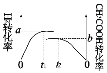
��a+b=_______���ﵽƽ���ʱ���С��ϵΪt1__________t2�����>����<������ȷ������
���𰸡�2H2(g)+2CO(g)![]() CH3COOH(l) ��H=+117.4kJ/mol BD 0.3mol/(L��min) ���ŷ�Ӧ�Ľ��з�Ӧ��Ũ�Ƚ��� 1.5 ��С ���� 100% ��ȷ��
CH3COOH(l) ��H=+117.4kJ/mol BD 0.3mol/(L��min) ���ŷ�Ӧ�Ľ��з�Ӧ��Ũ�Ƚ��� 1.5 ��С ���� 100% ��ȷ��
��������
��1����д��ȼ���ȵ��Ȼ�ѧ����ʽ��Ȼ����ݸ�˹���ɽ��м��㣻
��2���������������ƽ���ƶ��Ĺ��ɽ��з�����
��3���ٸ���v=![]() ���м��㣻
���м��㣻
�ڸ���ƽ�ⳣ��![]() ���м��㣻
���м��㣻
��ֻ����һ�ַ�Ӧ�ƽ�������ƶ���n(CH3COOH)���������С��n(��)��������ȣ���![]() ��С��H2��COͬ�ȳ̶ȵ�����Ũ�ȣ��൱�ڼ�ѹ����ƽ�������ƶ���
��С��H2��COͬ�ȳ̶ȵ�����Ũ�ȣ��൱�ڼ�ѹ����ƽ�������ƶ���
��4��һ���¶��£��ݻ�Ϊ1L�������ܱ�����֮�зֱ�ͨ��2molH2��2molCO��1molCH3COOH(g)���ֱ����������������ƽ�⣬���ն��ߴﵽ��Чƽ��״̬��������������ķ�Ӧ��ת����֮��Ϊ100%������������Ӧ�ֱ����������ʼ����Ӧ��Ũ�Ȳ�ͬ����ϵѹǿҲ��ͬ��������϶࣬�ݴ˷�����
��1�����ݷ�Ӧ����Ϣ�ã�
��2CO(g)+O2(g)=2CO2(g) ��H=��283.0��2kJ/mol��
��2H2(g)+O2(g)=2H2O(g) ��H=��285.8��2kJ/mol��
��CH3COOH(l)+2O2(g)=2CO2(g)+2H2O(l) ��H=��1255.0kJ/mol��
��ʽ+��ʽ����ʽ���ɵ�Ŀ�귽��ʽ��
2H2(g)+2CO(g)![]() CH3COOH(l) ��H=+117.4kJ/mol��
CH3COOH(l) ��H=+117.4kJ/mol��
�ʴ�Ϊ��2H2(g)+2CO(g)![]() CH3COOH(l) ��H=+117.4kJ/mol��
CH3COOH(l) ��H=+117.4kJ/mol��
��2��A�����º���ͨ��������壬��Ӱ������Ҳ��Ӱ��ƽ�⣬A����
B����ʱ������������ᣬ���Խ����������Ũ�ȣ�ʹƽ�������ƶ���CH3COOH�IJ�������B��ȷ��
C����Ӵ���ֻӰ�����ʲ�Ӱ��ƽ�⣬C����
D��ѹ�������ƽ���������������С�����ƶ���CH3COOH�IJ�������D��ȷ��
��ѡBD��
��3����0~2min����CO��ʾ��Ӧ����=![]() �����ŷ�Ӧ�Ľ��з�Ӧ��Ũ�ȼ�С��������С��
�����ŷ�Ӧ�Ľ��з�Ӧ��Ũ�ȼ�С��������С��
�ڻ�ѧƽ�ⳣ����ƽ��ʱ������Ũ���ݵij˻��뷴Ӧ��Ũ���ݵij˻��ı�ֵ���ʴ��¶���ƽ�ⳣ��![]() ��
��
��ֻ����һ�ַ�Ӧ�ƽ�������ƶ���n(CH3COOH)���������С��n(��)��������ȣ���![]() ��С��H2��COͬ�ȳ̶ȵ�����Ũ�ȣ��൱�ڼ�ѹ����ƽ�������ƶ�����CH3COOH�����������
��С��H2��COͬ�ȳ̶ȵ�����Ũ�ȣ��൱�ڼ�ѹ����ƽ�������ƶ�����CH3COOH�����������
�ʴ�Ϊ��0.3mol/(L��min)�����ŷ�Ӧ�Ľ��з�Ӧ��Ũ�Ƚ��� ��1.5����С ������
��4��һ���¶��£��ݻ�Ϊ1L�������ܱ�����֮�зֱ�ͨ��2molH2��2molCO��1molCH3COOH(g)���ֱ����������������ƽ�⣬���ն��ߴﵽ��Чƽ��״̬��������������ķ�Ӧ��ת����֮��Ϊ100%������������Ӧ�ֱ����������ʼ����Ӧ��Ũ�Ȳ�ͬ����ϵѹǿҲ��ͬ��������϶࣬�ʷ�Ӧ���ʲ�֪�ĸ���Ӧ�죬�ʴﵽƽ���ʱ�䲻ȷ����
�ʴ�Ϊ��100% ����ȷ����

 ��У����ϵ�д�
��У����ϵ�д�