��Ŀ����
����Ŀ������ʵʩ����ɽ��ˮ�������������о���������������
��1��H2�ڴ��������¿ɽ�NO��ԭΪN2����ͼ�Ǹ÷�Ӧ����1 molˮ�����������仯ʾ��ͼ��д���÷�Ӧ���Ȼ�ѧ����ʽ__________��

��2��2NO(g)��O2(g)![]() 2NO2(g)�ķ�Ӧ�������£�
2NO2(g)�ķ�Ӧ�������£�
��Ӧ��2NO(g)![]() N2O2(g)(��) ��H1��0��v1����k1����c2(NO)��v1����k1����c(N2O2)��
N2O2(g)(��) ��H1��0��v1����k1����c2(NO)��v1����k1����c(N2O2)��
��Ӧ��N2O2(g)��O2(g)![]() 2NO2(g����������H2��0��v2����k2����c(N2O2)��c(O2)��v2����k2����c2(NO2)��
2NO2(g����������H2��0��v2����k2����c(N2O2)��c(O2)��v2����k2����c2(NO2)��
��һ�������£���Ӧ2NO(g)��O2(g)![]() 2NO2(g)�ﵽƽ��״̬��ƽ�ⳣ��K��___________���ú�k1����k1����k2����k2���Ĵ���ʽ��ʾ������Ӧ��Ļ��E��___________������>����<��������������Ӧ��Ļ��E����
2NO2(g)�ﵽƽ��״̬��ƽ�ⳣ��K��___________���ú�k1����k1����k2����k2���Ĵ���ʽ��ʾ������Ӧ��Ļ��E��___________������>����<��������������Ӧ��Ļ��E����
����֪��Ӧ���ʳ���k���¶����߶������������¶Ⱥ�k2������ı���__________��������������С����������������k2������ı�����
��3���ҹ���ѧ������Ȼ�������о�����ȡ�����½�չ��������ͼװ�ÿɷ�����Ӧ��H2S��O2=H2O2��S����
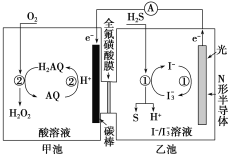
��װ����H����_______��Ǩ�ơ�
���ҳ���Һ�з�����Ӧ�����ӷ���ʽ��_______��
��4����ˮ����ʱ��ͨH2S(���S2��)��ʹijЩ�����������ɼ����ܵ��������ȥ��25 �棬ij��Һ��c(Mn2��)��0.02 mol��L��1�����ڷ�Һ��pHʹMn2����ʼ����ΪMnSʱ����Һ��c(H2S)��0.1 mol��L��1����ʱpHԼΪ_______����֪��Ksp��MnS����5.0��10��14��H2S�ĵ��볣����Ka1��1.5��10��7��Ka2��6.0��10��15��lg 6��0.8����
���𰸡�2H2(g)��2NO(g)=N2(g)��2H2O(g)����H��2(E1��E2)kJ��mol��1 ��2H2(g)��2NO(g)=N2(g)��2H2O(g)����H����2(E2��E1)kJ��mol��1��H2(g)��NO(g)=![]() N2(g)��H2O(g)����H��(E1��E2)kJ��mol��1��H2(g)��NO(g)=
N2(g)��H2O(g)����H��(E1��E2)kJ��mol��1��H2(g)��NO(g)=![]() N2(g)��H2O(g)�� ��H����(E2��E1)kJ��mol��1
N2(g)��H2O(g)�� ��H����(E2��E1)kJ��mol��1 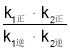 �� �� �� H2S��I3-=3I����S����2H�� 5.2
�� �� �� H2S��I3-=3I����S����2H�� 5.2
��������
(1)���������仯ͼ����Ӧ��=����Ӧ�Ļ��-�淴Ӧ�Ļ�ܣ��ٸ����Ȼ�ѧ��Ӧ����ʽ����д���
(2)��2NO(g)N2O2(g)�٣���N2O2(g)+O2(g)2NO2(g)�ڣ����ݸ�˹���ɣ���+�ڵ�2NO(g)+O2(g)2NO2(g)��ƽ�ⳣ��K=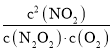 ���ɷ�Ӧ��ƽ��״̬������v1��=v1����v2��=v2��������v1����v2��=v1����v2������Ϊ����2NO(g)+O2(g)2NO2(g)���ʵ��Ƿ�Ӧ�ڣ����Է�Ӧ�ٵĻ��E1ԶС�ڷ�Ӧ�ڵĻ��E2��
���ɷ�Ӧ��ƽ��״̬������v1��=v1����v2��=v2��������v1����v2��=v1����v2������Ϊ����2NO(g)+O2(g)2NO2(g)���ʵ��Ƿ�Ӧ�ڣ����Է�Ӧ�ٵĻ��E1ԶС�ڷ�Ӧ�ڵĻ��E2��
�ڸ���Ӧ��Ϊ���ȷ�Ӧ���¶�����ƽ�����������ƶ�������
(3)��ԭ���������������������
�����ҳ��У�����ʧ�����������ʣ��ⵥ�ʵõ�������I���������ʵı仯ȷ�������ķ�Ӧ��
(4)��Qc=Ksp(MnS)ʱ��ʼ�������ɴ���������ӵ�Ũ�ȣ����Ka1��Ka2=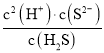 =1.5��10-7��6.0��10-15=9.0��10-22�����c(H+)��Ȼ��ɼ������Һ��pH��
=1.5��10-7��6.0��10-15=9.0��10-22�����c(H+)��Ȼ��ɼ������Һ��pH��
(1)����Ӧ���ΪE3��E2 ���淴Ӧ���ΪE3��E1������1 molˮ�������÷�Ӧ�ķ�Ӧ��Ϊ��H��(E3��E2)��(E3��E1)��(E1��E2)kJ��mol��1�����Ը÷�Ӧ���Ȼ�ѧ����ʽ��H2(g)��NO(g)=![]() N2(g)��H2O(g)����H��(E1��E2)kJ��mol��1��Ϊ2H2(g)��2NO(g)=N2(g)��2H2O(g) ��H��2(E1��E2) kJ��mol��1��
N2(g)��H2O(g)����H��(E1��E2)kJ��mol��1��Ϊ2H2(g)��2NO(g)=N2(g)��2H2O(g) ��H��2(E1��E2) kJ��mol��1��
(2)�٢���2NO(g) N2O2(g)(��)��v1����k1����c2(NO)��v1����k1����c(N2O2) ��H1��0��
����N2O2(g)��O2(g) 2NO2(g)(��)��v2����k2����c(N2O2)��c(O2)��v2����k2����c2(NO2)����H2��0���ɷ�Ӧ��ƽ��״̬������v1����v1����v2����v2��������v1����v2����v1����v2������k1����c2(NO)��k2����c(N2O2)��c(O2)��k1����c(N2O2)�� k2����c2(NO2)������K��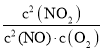 ��
��![]() ����Ϊ����2NO(g)��O2(g) 2NO2(g)���ʵ��Ƿ�Ӧ�������Է�Ӧ���Ļ��E��ԶС�ڷ�Ӧ���Ļ��E����
����Ϊ����2NO(g)��O2(g) 2NO2(g)���ʵ��Ƿ�Ӧ�������Է�Ӧ���Ļ��E��ԶС�ڷ�Ӧ���Ļ��E����
��k���¶����߶������������¶Ⱥ�k2������ı���С��k2������ı�����
(3)����ʾ��ͼ�п�������������̼��һ�����ü�Ϊ�����������Ӵ��ҳ�����׳أ�
���ҳ���Һ�У�������I3-����������ԭ��Ӧ������ʧ���ӱ�Ϊ���ʣ�I3-�õ��ӱ�ΪI�������ӷ�ӦΪH2S��I3-=3I����S����2H����
(4)25 ����ij��Һ��c(Mn2��)��0.02 mol��L��1�����ڷ�Һ��pHʹMn2����ʼ����ΪMnSʱ��c(S2��)��![]() ��
��![]() ��2.5��10��12 mol��L��1��
��2.5��10��12 mol��L��1��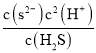 ��Ka1��Ka2��c(H��)��
��Ka1��Ka2��c(H��)��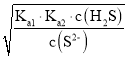 ��
��![]() ��6��10��6 mol��L��1��pH��5.2��
��6��10��6 mol��L��1��pH��5.2��

����Ŀ��25 �棬������ĵ���ƽ�ⳣ�����±���
Ka1 | Ka2 | |
H2SO3 | 1.3��10��2 | 6.3��10��8 |
H2CO3 | 4.2��10��7 | 5.6��10��11 |
(1)HSO![]() �ĵ���ƽ�ⳣ������ʽK��______________________________��
�ĵ���ƽ�ⳣ������ʽK��______________________________��
(2)H2SO3��Һ��NaHCO3��Һ��Ӧ����Ҫ���ӷ���ʽΪ_______________��
����Ŀ��CO��H2����Ϊ�ϳ������úϳ������Ժϳ����ᡣ�ش��������⣺
(1)��֪CO��H2��CH3COOH��ȼ������H�ֱ�Ϊ-283.0kJ/mol��-285.8kJ/mol��-1255.0kJ/mol�����úϳ����ϳ�CH3COOH(l)�Ŀ�����̵��Ȼ�ѧ��Ӧ����ʽΪ___________________________________________��
(2)���ܱ������з����ϳ�����ķ�Ӧ�����п������CH3COOH���ʵĴ�ʩ��________��
A�����º��ݣ�ͨ���������� B.��ʱ�������������
C�������Ч���� D��ѹ���������
(3)��150��ʱ��2L���ܱ������з�����Ӧ��
2H2(g)+2CO(g)![]() CH3COOH(g) ��H>0����ʼͨ��4molH2��4molCO��CH3COOH������Ũ��������ʱ��仯���±���ʾ��
CH3COOH(g) ��H>0����ʼͨ��4molH2��4molCO��CH3COOH������Ũ��������ʱ��仯���±���ʾ��
ʱ��/min | 0 | 2 | 4 | 6 | 8 |
c(CH3COOH)/mol/L | 0 | 0.3 | 0.5 | 0.6 | 0.6 |
��0~2min����CO��ʾ�÷�Ӧ������Ϊ_________________�����ŷ�Ӧ�Ľ������������Ŀ���ԭ����____________________________________________________��
��150��ʱ�÷�Ӧ��ƽ�ⳣ������ֵԼΪ_________��������������һλС����
��ƽ�����ͨ��1molH2����CH3COOH(g)���������________�����ٴ�ͨ��1molH2��1molCO����CH3COOH(g)���������_________�����������С�����ߡ����䡱��
(4)һ���¶��£��ݻ���Ϊ1L�������ܱ�����֮�н�������ʵ�飺
ʵ���� | ��ʼͶ�� |
�� | 2molH2��2molCO |
�� | 1molCH3COOH(g) |
��ʵ�����H2��ʵ�����CH3COOH��ת������ʱ��仯ͼʾ���£�
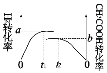
��a+b=_______���ﵽƽ���ʱ���С��ϵΪt1__________t2�����>����<������ȷ������