��Ŀ����
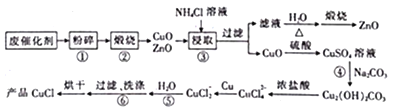
����Ŀ����֪t ��ʱAgCl��Ksp��4��10��10����t ��ʱ��Ag2CrO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ������˵���������(����)
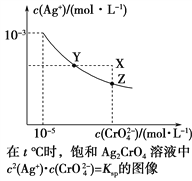
A. ��t ��ʱ��Ag2CrO4��KspΪ1��10��11
B. �ڱ�����Һ�м���K2CrO4(s)��ʹ��Һ��Y�㵽Z��
C. ��t ����Ag2CrO4(s)��2Cl��(aq) ![]() 2AgCl(s)��CrO
2AgCl(s)��CrO![]() (aq)ƽ�ⳣ��K��6.25��107
(aq)ƽ�ⳣ��K��6.25��107
D. ��t ��ʱ����0.001 mol��L��1 AgNO3��Һ�ζ�20 mL 0.001 mol��L��1 KCl��0.001 mol��L��1��K2CrO4�Ļ����Һ��CrO![]() �ȳ���
�ȳ���
���𰸡�D
��������A.��t ��ʱ��Ag2CrO4��KspΪKsp(Ag2CrO4)=c2(Ag+)c(CrO42-)=(1��10��3)2��10��5=1��10��11��A��ȷ��B. �ڱ�����Һ�м���K2CrO4(s)��c(CrO42-)����ʹ��Һ��Y�㵽Z�㣬��B��ȷ��C. ��t ����Ag2CrO4(s)��2Cl��(aq) ![]() 2AgCl(s)��CrO
2AgCl(s)��CrO![]() (aq)��ƽ�ⳣ��K��
(aq)��ƽ�ⳣ��K��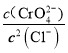 ��
��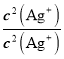 =
=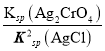 =6.25��107����C��ȷ��D. ��t ��ʱ����ʼ����AgCl����ʱ��c(Ag+)=
=6.25��107����C��ȷ��D. ��t ��ʱ����ʼ����AgCl����ʱ��c(Ag+)=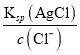 =4��10��7mol/L����ʼ����Ag2CrO4����ʱ��[
=4��10��7mol/L����ʼ����Ag2CrO4����ʱ��[![]() =1��10��4mol/L������Cl-������Ҫ��c(Ag+)��С���ȳ�������D����ѡD��
=1��10��4mol/L������Cl-������Ҫ��c(Ag+)��С���ȳ�������D����ѡD��

 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д� ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д� �㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�㽭֮�ǿ�ʱ�Ż���ҵϵ�д�����Ŀ���������̼��������ϳɻ���ԭ�ϣ��ȿ��Լ��ٻ�����Ⱦ������ЧӦ�����ܱ��Ϊ����
��CO2���ۺ������ǽ������ЧӦ����Դ�������Ч;����
(1)O2��H2�ڴ��������¿ɷ�����Ӧ����CH3OH����֪CH3OH��H2��ȼ���ȷֱ�Ϊ��H1=-akJ��mol-1����H2=-bkJ��mol-1����1molˮ����ת��ΪҺ̬ˮʱ�ų�ckJ��������
��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)��H=___________kJ��mol-1��
CH3OH(g)+H2O(g)��H=___________kJ��mol-1��
(2)����CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)������CO2��H2��ʼͶ�ϱ�Ϊ1��3ʱ���¶ȶ�CO2ƽ��ת���ʼ��״����ʵ�Ӱ����ͼ��ʾ����ͼ��֪��ȡCH3OH�����˵��¶���___________���������������CO2ת��ΪCH3OH��ƽ��ת���ʵĴ�ʩ��___________��
CH3OH(g)+H2O(g)������CO2��H2��ʼͶ�ϱ�Ϊ1��3ʱ���¶ȶ�CO2ƽ��ת���ʼ��״����ʵ�Ӱ����ͼ��ʾ����ͼ��֪��ȡCH3OH�����˵��¶���___________���������������CO2ת��ΪCH3OH��ƽ��ת���ʵĴ�ʩ��___________��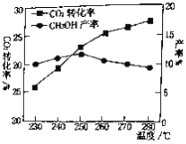
A��ʹ�ô��� B��������ϵѹǿ
C������CO2��H2�ij�ʼͶ�ϱ� D��Ͷ�ϱȲ��������������䣬���ӷ�Ӧ���Ũ��
��CO�Ǻϳ����ء������ԭ�ϡ�
(3)�ϳ����صķ�Ӧ��2NH3(g)+CO(g)![]() CO(NH2)2(g)+H2(g)��H=-81.0kJ��mol-1��
CO(NH2)2(g)+H2(g)��H=-81.0kJ��mol-1��
��T��ʱ�������Ϊ2L�ĺ����ܱ������У���2molNH3��1molCO��Ϸ�����Ӧ��5minʱ��NH3��ת����Ϊ80%����0��5min�ڵ�ƽ����Ӧ����Ϊv(CO)=___________��
����֪��
�¶�/K | 398 | 498 | �� |
ƽ�ⳣ��/K | 126.5 | K1 | �� |
��K1___________126.5(�������<��);���������___________��
(4)ͨ���˹�������ÿɽ�COת����HCOOH��
����֪�����£�Ũ�Ⱦ�Ϊ0.1mol��L-1��HCOOH��HCOONa�����ҺpH=3.7,��HCOOH�ĵ��볣��Ka��ֵΪ___________ (��֪lg2=0.3)��
���õ绯ѧ������HCOOH��ˮ����ɵ���Ⱦ����ԭ���ǵ��CoSO4��ϡ�����HCOOH�����Һ���õ�������Co3+��HCOOH������CO2��Co3+����HCOOH�����ӷ���ʽΪ___________;��������仯�����ǰ��Co2+��Ũ�Ƚ�___________ (�������С�����䡱)��