��Ŀ����
16�� ʵ������ͼʾװ����ȡ����������
ʵ������ͼʾװ����ȡ������������1��ŨH2SO4�������ǣ��ٴ���������ˮ����
��2���ұ��ռ��Թ���װ�б���̼������Һ������Һ���������ܽ��Ҵ���ȥ�ӷ�������������Ҵ�����С���������ܽ�ȣ������ڷֲ�
����Һ��ֲ㣻�����ܲ��ܲ���Һ�����£�ԭ���Ƿ�ֹ����������ǰ�����Թ��м��뼸�����Ƭ�������Ƿ�ֹ���У�
��3���Ҵ���ͭ������������ȩ�ķ�Ӧ����ʽΪ2C2H5OH+O2 $��_{��}^{Cu}$ 2CH3CHO+2H2O
��4����ȡ���������ķ�Ӧ����ʽ��CH3COOH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOC2H5+H2O��
���� ��1��Ũ���������ˮ�ԣ�ǿ�����ԣ���Ϸ�Ӧ���ɽ��
��2������̼������Һ�����ᷴӦ��ȥ���ᡢͬʱ���������������ܽ�ȣ����ڷֲ㣻��������Һ���¿��ܷ���������Һ�����Ҫ�����Ƭ����ֹ���У�
��3���Ҵ�������������ȩ��ˮ��
��4��������Ӧ�ı���Ϊ�����ǻ��������⣬�÷�Ӧ��������������ˮ����Ϊ���淴Ӧ��
��� �⣺��1���������Ҵ�����������Ӧ����Ũ�������������÷�ӦΪ���淴Ӧ��Ũ������ˮ����ƽ���������������������ƶ���Ũ���������Ϊ�����ã���ˮ���ã�
�ʴ�Ϊ����������ˮ����
��2���Ʊ���������ʱ���ñ���̼������Һ��Ŀ�����кͻӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У�������������������ζ���ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ��������ܲ��ܲ�����Һ�У�����Ҫ���ڱ���̼������Һ��Һ���ϣ�����Һ���¿��ܷ���������������������ʱ�Ƚ�ʢ�л������Թܳ�����ñ���̼������Һ�кͻӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У��ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����÷ֲ��ȡ�ϲ������������Һ�������Ҵ��е�ͣ�����Ҫ�����Ƭ����ֹ���У�
�ʴ�Ϊ������̼������Һ����ȥ�ӷ�������������Ҵ�����С���������ܽ�ȣ������ڷֲ㣻��ֹ��������ֹ���У�
��3���Ҵ�������������ȩ��ˮ���䷴Ӧ����ʽΪ2C2H5OH+O2 $��_{��}^{Cu}$ 2CH3CHO+2H2O���ʴ�Ϊ��2C2H5OH+O2 $��_{��}^{Cu}$ 2CH3CHO+2H2O��
��4��������Ӧ�ı���Ϊ�����ǻ��������⣬�������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ���÷�ӦΪ���淴Ӧ����ΪCH3COOH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOC2H5+H2O��
�ʴ�Ϊ��CH3COOH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOC2H5+H2O��
���� ���⿼���������������Ʊ���ע����������������Ʊ�ԭ����ʵ�鷽���������������������ᡢ�Ҵ����ʵ�������Ŀ�ѶȲ���

 ��ѧ����ϵ�д�
��ѧ����ϵ�д� �ο�������ϵ�д�
�ο�������ϵ�д�| A�� |  | B�� |  | C�� | 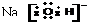 | D�� |  |
| A�� | �٢ڢۢ� | B�� | �ڢ٢ۢ� | C�� | �ڢܢ٢� | D�� | �ۢܢ٢� |
 ��ijʵ��С���H2O2�ķֽ���������̽�����±��Ǹ�ʵ��С���о�H2O2�ֽ����ʵ�����ʱ��¼��һ�����ݣ���������ͬ��״̬��ͬ��MnO2�ֱ����ʢ��15mL 5%��H2O2��Һ�Ĵ��Թ��У����ô����ǵ�ľ�����ԣ�������£�
��ijʵ��С���H2O2�ķֽ���������̽�����±��Ǹ�ʵ��С���о�H2O2�ֽ����ʵ�����ʱ��¼��һ�����ݣ���������ͬ��״̬��ͬ��MnO2�ֱ����ʢ��15mL 5%��H2O2��Һ�Ĵ��Թ��У����ô����ǵ�ľ�����ԣ�������£�| MnO2 | �����Թ���� | �۲��� | ��Ӧ��������ʱ�� |
| ��ĩ״ | ���� | ���ҷ�Ӧ�������ǵ�ľ����ȼ | 3.5min |
| ��״ | �� | ��Ӧ���������Ǻ�����ľ��δ��ȼ | 30min |
��2��ʵ���������������Ĵ�Ч����Ӵ�������йأ�
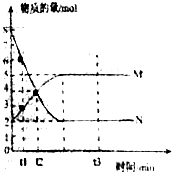
��һ���¶��£����ݻ�ΪVL���ܱ������н��з�Ӧ��aN��g��?bM��g����M��N�����ʵ�����ʱ��ı仯������ͼ��ʾ��
��1���˷�Ӧ�Ļ�ѧ����ʽ��$\frac{a}{b}$=$\frac{2}{1}$
��2��t1��t2ʱ�̣���M��Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ��$\frac{1}{��{t}_{2}-{t}_{1}��V}$mol•L-1•min-1
��3��ƽ��ʱ��N��ת����Ϊ75%��
��4��������������˵��������Ӧ�ﵽƽ��״̬����CE
A����Ӧ��M��N�����ʵ���֮��Ϊ1��1
B��������������������ʱ��ı仯���仯
C���������������ʵ�������ʱ��ı仯���仯
D����λʱ����ÿ����amolN��ͬʱ����bmolM
E����������ѹǿ����ʱ��ı仯���仯��
| A�� | X����̬�⻯����ȶ��Աȼ���ǿ | B�� | X�������������XO2 | ||
| C�� | XӦΪ�ǽ���Ԫ�� | D�� | X������������ˮ������ǿ�� |

 CH3COOCH2CH2CH3+H2O��
CH3COOCH2CH2CH3+H2O��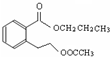 ��
�� ��
��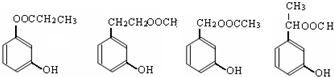 ��
��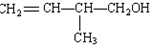
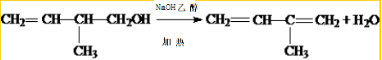 ��
��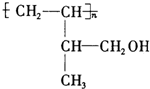 ��
��