��Ŀ����
����Ŀ���Ϸ���ij���л�ѧ�����Կγ�ѧϰС���������⣨Fe2O3������ϵ��ʵ�飬����֮��Ĺ�ϵͼ���¡�
������ѧ֪ʶ�ش��������⣺
IHCl������
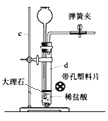
ʵ�������ܶ�Ϊ1.18g/mL����������Ϊ36.5%Ũ��������480mL0.1mol/L��������Һ��
��1������480mL0.1mol/L��������Һ��ҪŨ��������Ϊ___mL��(����2λ��Ч����)
��2�������ձ�������������Ͳ����ͷ�ιܺ��Լ�ƿ����Ҫ��������___��
��3�������������������������ҺŨ�Ƚ��к�Ӱ�죿(�ƫ�͡���ƫ�ߡ�)
δϴ���ձ�___������ʱ���ӿ���____��
II̽��ʵ��
��1��д����A���뵽��ˮ���Ʊ�B�Ļ�ѧ����ʽ��___
��2������˵����ȷ����___������ţ���
��Bת����C�����˻�ѧ��Ӧ
����A�Ʊ�B������Խ��Խ��
������B���ж����ЧӦ
�ܰ�B��C��ɵĻ������ˣ���Һ����ɫ��
���������̶����漰��������ԭ��Ӧ
��3������ɫ����C�м���NaClO��NaOH�����Һ������һ�ָ�Чɱ����ˮ��Na2FeO4����֪ÿ����0.2mol��Na2FeO4����0.3molNaClO����÷�Ӧ�Ļ�ԭ����Ϊ___��
���𰸡�4.2 500mL����ƿ ƫ�� ƫ�� FeCl3+3H2O![]() Fe(OH)3�����壩+3HCl �ۢ� NaCl
Fe(OH)3�����壩+3HCl �ۢ� NaCl
��������
I.��1��������������Һ�����ѡ����ʹ�������ƿ������![]() ����Ũ��������ʵ���Ũ�ȣ���������Һϡ���������ʵ����ʵ������������ҪŨ����������
����Ũ��������ʵ���Ũ�ȣ���������Һϡ���������ʵ����ʵ������������ҪŨ����������
��2��������Ũ��Һ����һ�����ʵ���Ũ�ȵ�ϡ��Һ��һ�㲽��ȷ��ʵ������������
��3���������������ʵ����ʵ�������Һ�����Ӱ�죬����![]() ������������
������������
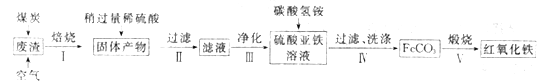
II. ���⣨Fe2O3�������������ᷴӦ���ɵ�AΪ�Ȼ������Ȼ��������ˮ�л���B�����������壬�Ȼ������������Ʒ�Ӧ������C��������������
��1�����Ȼ��������ˮ���Ʊ����������������õ����Ȼ�����ˮ�⣻
��2����Bת����CΪ����ľ۳���
�ڽ��Ȼ��������ˮ���Ʊ�������������ʱ������ʱ�������ʹ����۳������ܳ�ʱ����ȣ�
�۽�����ж����ЧӦ��
����������������ͨ����ֽ������������������ͨ����ֽ��
�ݸ���Ԫ�ػ��ϼ��Ƿ�仯�ж��Ƿ����������ԭ��Ӧ��
��3��������Ϣ��֪Fe(OH)3ʧ���ӱ���������Na2FeO4��NaClO���������ԣ����������������õ��ӣ���ClԪ�صõ��Ӻ�Ļ��ϼ�Ϊx�����ݵ�ʧ�����غ����x��ֵ���Ӷ�ȷ����ԭ���
I.��1������480mL0.1mol/L��������Һ��Ӧѡ��500mL����ƿ���ܶ�Ϊ1.18g/mL����������Ϊ36.5%Ũ�������ʵ���Ũ��![]() ������ҪŨ�������ΪV����������Һϡ���������ʵ����ʵ������䣬�ɵ�11.8mol/L��V=0.5L��0.1mol/L�����V=4.2mL��
������ҪŨ�������ΪV����������Һϡ���������ʵ����ʵ������䣬�ɵ�11.8mol/L��V=0.5L��0.1mol/L�����V=4.2mL��
�ʴ�Ϊ��4.2��
��2����Ũ��������ϡ�����ʵ�鲽����������㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ�ȣ����ʵ�鲽���֪��ʵ���������������ձ�������������Ͳ����ͷ�ιܺ��Լ�ƿ����Ҫ��������500mL����ƿ��
�ʴ�Ϊ��500mL����ƿ��
��3��δϴ���ձ��������ʻ���ʧ��ʹ���Ƶ���ҺŨ��ƫ�ͣ�����ʱ���ӿ��ߣ���ʹ���������ˮ�����ƫС����ʹ���Ƶ���Һ��Ũ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ͣ�ƫ�ߣ�
II. ���⣨Fe2O3�������������ᷴӦ���ɵ�AΪ�Ȼ������Ȼ��������ˮ�л���B�����������壬�Ȼ������������Ʒ�Ӧ������C��������������
��1�����Ȼ��������ˮ���Ʊ�������������Ļ�ѧ����ʽ�ǣ�FeCl3+3H2O![]() Fe(OH)3�����壩+3HCl��
Fe(OH)3�����壩+3HCl��
�ʴ�Ϊ��FeCl3+3H2O![]() Fe(OH)3�����壩+3HCl��
Fe(OH)3�����壩+3HCl��
��2����Bת����CΪ����ľ۳�������ľ۳��������仯���̣��ʢٴ���
�ڽ��Ȼ��������ˮ���Ʊ�������������ʱ������ʱ�������ʹ����۳������ܳ�ʱ����ȣ��ʢڴ���
����������������ж����ЧӦ���ʢ���ȷ��
�ܰ�����������������������������ˣ���������������ͨ����ֽ����Һ�Ǻ��ɫ���ʢܴ���
��������Ӧ����û��Ԫ�ػ��ϼ۵ı仯����������ԭ��Ӧ���ʢ���ȷ��
�����������ۢ���ȷ��
�ʴ�Ϊ���ۢݣ�
��3��������Ϣ��֪Fe(OH)3ʧ���ӱ���������Na2FeO4��NaClO���������ԣ����������������õ��ӣ���ClԪ�صõ��Ӻ�Ļ��ϼ�Ϊx�����ݵ�ʧ�����غ�ɵ�![]() ����ã�x=-1����÷�Ӧ�Ļ�ԭ����ΪNaCl��
����ã�x=-1����÷�Ӧ�Ļ�ԭ����ΪNaCl��
�ʴ�Ϊ��NaCl��

����Ŀ���������ʵ����ѡ���װ�û�����(�г�װ������ȥ)��ȷ���ǣ� ��
A | B | C | D | |
ʵ�� | ��ȡ����������CO2���� | ��CCl4��ȡ��ˮ�е�Br2 | ��ȥCO2��������HCl | ����NaCl������Һ�Ʊ�NaCl���� |
װ�û����� |
|
|
|
|
A.AB.BC.CD.D