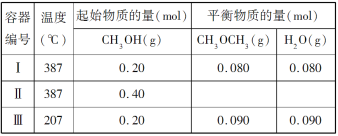
��Ŀ����
����Ŀ���Ժ��ܷϴ�������Ҫ�ɷ�ΪCo��Fe��SiO2��Ϊԭ�ϣ���ȡ�����ܵ��������£�
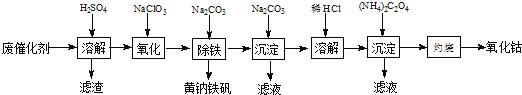
��1���ܽ⣺�ܽ����ˣ�������ϴ��2��3�Σ�ϴҺ����Һ�ϲ�����Ŀ����_________________��������������Ҫ�ɷ���_________��д��ѧʽ����
��2�����������Ƚ��������¼���NaClO3����Fe2+������Fe3+�������ӷ���ʽ_________________��
��֪�����軯�ػ�ѧʽΪK3[Fe(CN)6]�������軯�ػ�ѧʽΪK4[Fe��CN��6]3H2O��
3Fe2++2[Fe(CN)6]3-=Fe3[Fe(CN)6]2������ɫ������
4Fe3++3[Fe(CN)6]4-=Fe4[Fe(CN)6]3������ɫ������
ȷ��Fe2+�Ƿ�������ȫ�ķ�����______________�����ɹ�ѡ����Լ������軯����Һ�������軯����Һ�����ۡ�KSCN��Һ��
��3������������������Na2CO3������ȣ����ɻ�������[Na2Fe6(SO4)4(OH)12]������д���÷�Ӧ�Ļ�ѧ����ʽ______________��
��4�����������ɳ�����ʽ̼����[(CoCO3)23Co(OH)2]��������ϴ�ӣ�ϴ�ӵIJ�����______________��
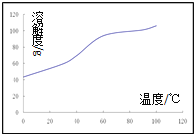
��5���ܽ⣺CoCl2���ܽ��������ͼ��ʾ���ʽ̼�����м�������ϡ���ᣬ���ȱ߽�������ȫ�ܽ������ȹ��ˣ���ԭ����______________��
��6�����գ�ȷ��ȡ����CoC2O41.470g���ڿ����г�����յ�0.830g�����ܣ�д�������ܵĻ�ѧʽ_________��
���𰸡�����ܵ�Ԫ�ص������� SiO2 6Fe2++6H++ClO3-�T6Fe3++Cl-+3H2O ȡ���������Һ�������Թ��У��μӼ������軯����Һ��������ɫ�������ɣ���Fe2+��ȫ���������� 3Fe2(SO4)3+6H2O+6Na2CO3=Na2Fe6(SO4)4(OH)12��+5Na2SO4+6CO2�� ��©���м�������ˮ����û����������ʹ��Һ�������ظ�����2��3�� ��ֹ���¶Ƚ��ͣ�CoCl2�������� Co2O3
��������
������ͼ��֪�����ܷϴ����м���ϡ���ᣬ�ܺ��������ᷴӦ����Ӧ�Ļ�ѧ����ʽΪCo+H2SO4=CoSO4+H2����Fe+H2SO4=FeSO4+H2��������������ϡ�����Ӧ�����ˣ������Dz��ܵĶ������裬����Һ�������ܡ��������������������Ļ����Һ������Һ�м��������ƣ������ƾ��������ԣ����������½��������������������ӣ���Ӧ����Һ�м���̼���ƣ�������ҺpHΪ2������Һ�е�������ת��Ϊ��������[Na2Fe6��SO4��4��OH��12]������ȥ�����ˣ�����Һ�м�������̼���ƣ�������ҺpHΪ7���õ�̼���ܳ��������ˣ��������ܽ�̼���ܳ���������������Һ�м������泥��õ������ܳ��������ˣ����ղ����ܳ����������ܳ����������������Ӧ���������ܣ��ݴ˷������
(1)ϴҺ����Һ�ϲ������ϴ�Ӻ���Һ���ܵ������ʣ���SiO2������ϡ���ᣬ����������Ҫ�ɷ���SiO2��
(2)�������ӱ���������������������ӣ�1molr����������ʧȥ1mol�ĵ��ӣ���1mol����������ӵõ�6mol�ĵ��ӣ����ݵ��ӵ�ʧ�غ㣬��֪���ӷ���ʽΪ��6Fe2++6H++ClO3-�T6Fe3++Cl-+3H2O��ȡ���������Һ�������Թ��У��μӼ������軯����Һ��������ɫ�������ɣ���Fe2+��ȫ����������
(3)������������̼���Ʒ���˫ˮ��õ�������������ѧ��Ӧ����ʽΪ��3Fe2(SO4)3+6H2O+6Na2CO3=Na2Fe6(SO4)4(OH)12��+5Na2SO4+6CO2����
(4)����ϴ�ӵķ�������©���м�������ˮ����û����������ʹ��Һ�������ظ�����2��3�Σ�(5)CoCl2���ܽ�����߿�֪�����¶ȵ����ߣ�CoCl2���ܽ���������Գ��ȹ��ˣ���ֹ�¶Ƚ����Ȼ���������
(6)CoC2O4������Ϊ1.470g������֪����Ϊ0.01mol��CoԪ������Ϊ0.59g��������������Ϊ0.83g������������Ԫ������Ϊ0.83g-0.59g=0.24g������������Coԭ����Oԭ�����ʵ���֮��Ϊ0.01mol��(0.24/16)��2��3����Co������ΪCo2O3��

����Ŀ����ѧ������������ء�
I.K2Cr2O7�����ڼ��˾���Ƿ�ƺ��ʻ��Cr2O72-(��ɫ)+CH3CH2OH��Cr3+(��ɫ)+CH3COOH(δ��ƽ��
��1����̬Crԭ�ӵļ۵��ӹ������ʽΪ__��
��2��CH3COOH����������Ԫ�صĵ縺���ɴ�С��˳��Ϊ__��̼ԭ�ӵĹ���ӻ�����Ϊ__��������������������Ŀ֮��Ϊ__��
��3����֪Cr3+�ȹ���Ԫ��ˮ�����ӵ���ɫ�����ʾ��
���� | Sc3+ | Cr3+ | Fe2+ | Zn2+ |
ˮ�����ӵ���ɫ | ��ɫ | ��ɫ | dz��ɫ | ��ɫ |
�����ԭ�ӽṹ�Ʋ�Sc3+��Zn2+��ˮ������Ϊ��ɫ��ԭ��Ϊ__��
II.ZnCl2Ũ��Һ�����ڳ�ȥ��������������������FeO��Ӧ�ɵ�Fe[Zn(OH)Cl2]2��Һ��
��4��Fe[Zn(OH)Cl2]2��Һ�в����ڵ�������������__(��ѡ����ĸ)��
A.���Ӽ� B.���ۼ� C.������ D.��λ�� E.���»��� F.���
��Һ��[Zn(OH)Cl2]-�ĽṹʽΪ__��
III.п������������Ԫ��֮һ����ѻ���ʽ��ͼ1�������ṹ��ͼ2��
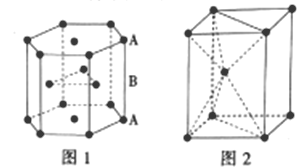
��5��п�Ķѻ���ʽΪ__����λ��Ϊ__��
��6����пԭ�ӵİ뾶Ϊapm�������ӵ�������ֵΪNA����п������ܶ�Ϊ___g/cm3(�ú�a�Ĵ���ʽ��ʾ)��