��Ŀ����
5����Ҫ�ľ�ϸ��ѧƷM��N����������������Ϳ�ϡ�ɱ����ȣ��ϳ�·������ͼ��ʾ��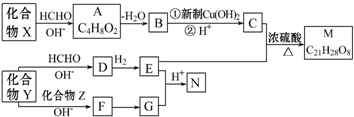
��֪��
i��
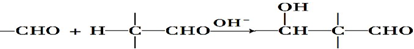
ii��RCHO+
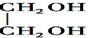 $\stackrel{H+}{��}$
$\stackrel{H+}{��}$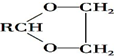 +H2O
+H2Oiii��N�Ľṹ��ʽ��

��ش��������⣺
��1��N�ķ���ʽΪC23H24O4
��2��A�к����������������ǻ���ȩ����
��3����A����B�ķ�Ӧ��������ȥ��Ӧ��
��4��X�Ľṹ��ʽ��CH3CH2CHO��
��5��C�ͼ״���Ӧ�IJ�����Ծۺ��γ��л��������þۺϷ�Ӧ�Ļ�ѧ����ʽ��
 ��
����6����Y����D�Ļ�ѧ����ʽ��
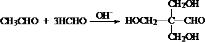 ��
����7������˵����ȷ����bc��
a��E�ܷ�����ȥ��Ӧ
b.1mol M���4mol����
c��X��Y��ͬϵ��
d.1mol G������H2�ӳɿ�����2mol H2
e��Z�Ľṹ��ʽ��
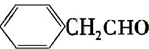
��8��E��ͬ���칹���ж��֣�д����������������ͬ���칹��Ľṹ��ʽ��
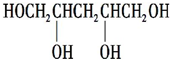 ��
��a����E������ͬ������ b����˴Ź���������5�ַ壨��ʾ��ͬһ̼ԭ���������������ϵ��ǻ��Dz��ȶ��ģ�
���� �л���X��HCHO������Ϣi�ķ�Ӧ�����A�ķ���ʽC4H8O2����֪XΪCH3CH2CHO��AΪHOCH2CH��CH3��CHO��A������ȥ��Ӧ��ȥ1����H2O����B����BΪCH2�TC��CH3��CHO��B��������CΪCH2�TC��CH3��COOH��
����Ϣiii��N�Ľṹ�������Ϣii�з�Ӧ��֪���γ�M������Ϊ ��
�� ��C��E��Ӧ���Ժϳ�M�����M�ķ���ʽC21H28O8��֪��EΪ
��C��E��Ӧ���Ժϳ�M�����M�ķ���ʽC21H28O8��֪��EΪ ����GΪ
����GΪ ��C��Eͨ��������Ӧ����M��
��C��Eͨ��������Ӧ����M��
��Y��E��ת�������E�Ľṹ��֪��Y�����������ٽ��G�Ľṹ��֪��������Z�к��б�������Ϸ�Ӧ��Ϣi��֪��YΪCH3CHO��ZΪ ����FΪ
����FΪ ��
��
��E�Ľṹ��֪��1����CH3CHO��3����HCHO������Ϣi��Ӧ����D��DΪ ��D�����������ӳɷ�Ӧ����E��
��D�����������ӳɷ�Ӧ����E�� �����ݴ˽��
�����ݴ˽��
��� �⣺�л���X��HCHO������Ϣi�ķ�Ӧ�����A�ķ���ʽC4H8O2����֪XΪCH3CH2CHO��AΪHOCH2CH��CH3��CHO��A������ȥ��Ӧ��ȥ1����H2O����B����BΪCH2�TC��CH3��CHO��B��������CΪCH2�TC��CH3��COOH��
����Ϣiii��N�Ľṹ�������Ϣii�з�Ӧ��֪���γ�M������Ϊ ��
�� ��C��E��Ӧ���Ժϳ�M�����M�ķ���ʽC21H28O8��֪��EΪ
��C��E��Ӧ���Ժϳ�M�����M�ķ���ʽC21H28O8��֪��EΪ ����GΪ
����GΪ ��C��Eͨ��������Ӧ����M��
��C��Eͨ��������Ӧ����M��
��Y��E��ת�������E�Ľṹ��֪��Y�����������ٽ��G�Ľṹ��֪��������Z�к��б�������Ϸ�Ӧ��Ϣi��֪��YΪCH3CHO��ZΪ ����FΪ
����FΪ ��
��
��E�Ľṹ��֪��1����CH3CHO��3����HCHO������Ϣi��Ӧ����D��DΪ ��D�����������ӳɷ�Ӧ����E��
��D�����������ӳɷ�Ӧ����E�� ����
����
��1����N�Ľṹ��ʽ��֪������ʽΪC23H24O4���ʴ�Ϊ��C23H24O4��
��2������������֪��AΪHOCH2CH��CH3��CHO�������ǻ���ȩ�����ʴ�Ϊ���ǻ���ȩ����
��3��A��B��HOCH2CH��CH3��CHO������ȥ��Ӧ��ȥ1����H2O����CH2�TC��CH3��CHO���ʴ�Ϊ����ȥ��Ӧ��
��4��������������֪��X�Ľṹ��ʽ��CH3CH2CHO���ʴ�Ϊ��CH3CH2CHO��
��5��CH2�TC��CH3��COOH�ͼ״���Ӧ�IJ���ΪCH2�TC��CH3��COOCH3��������ͨ���Ӿ۷�Ӧ���ɸ߾����Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��6��Y����D��1����CH3CHO��3����HCHO������Ϣi��Ӧ���� ����Ӧ�Ļ�ѧ����ʽ��
����Ӧ�Ļ�ѧ����ʽ��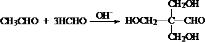 ��
��
�ʴ�Ϊ��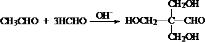 ��
��
��7��a��EΪ �����ǻ�������̼ԭ�����ڵ�̼ԭ����û��Hԭ�ӣ����ܷ�����ȥ��Ӧ����a����
�����ǻ�������̼ԭ�����ڵ�̼ԭ����û��Hԭ�ӣ����ܷ�����ȥ��Ӧ����a����
b�� ��CH2�TC��CH3��COOH������ȫ������Ӧ����M��1molM�к���4mol��������b��ȷ��
��CH2�TC��CH3��COOH������ȫ������Ӧ����M��1molM�к���4mol��������b��ȷ��
c��XΪCH3CH2CHO��YΪCH3CHO�����ߺ�����ͬ�Ĺ����š�����Ϊ������ṹ���ƣ����1��CH2ԭ���ţ���Ϊͬϵ���c��ȷ��
d��GΪ ��������̼̼˫����ȩ���������������ӳɷ�Ӧ��1mol G������H2�ӳɿ�����5mol H2����d����
��������̼̼˫����ȩ���������������ӳɷ�Ӧ��1mol G������H2�ӳɿ�����5mol H2����d����
e��Z�Ľṹ��ʽ�� ����e����
����e����
�ʴ�Ϊ��bc��
��8��E�� ����ͬ���칹���ж��֣���������������ͬ���칹��Ľṹ��ʽ��a����E������ͬ�����ţ�b����˴Ź���������5�ַ壨ͬһ̼ԭ���������������ϵ��ǻ��Dz��ȶ��ģ�����ͬ���칹��Ϊ��
����ͬ���칹���ж��֣���������������ͬ���칹��Ľṹ��ʽ��a����E������ͬ�����ţ�b����˴Ź���������5�ַ壨ͬһ̼ԭ���������������ϵ��ǻ��Dz��ȶ��ģ�����ͬ���칹��Ϊ��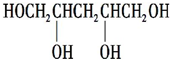 ��
��
�ʴ�Ϊ��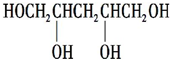 ��
��
���� ���⿼���л��ƶ���ϳɣ�������ø����ķ�Ӧ��Ϣ���л���Ľṹ������ʽȷ��X��E��G�Ľ���ǹؼ����ϺõĿ���ѧ������˼ά��������ѧ�������ѶȽϴ�

| A�� | Al3+��Na+��NO3-��Cl- | B�� | K+��Na+��NO3-��Cl- | ||
| C�� | K+��Na+��Cl-��AlO2- | D�� | K+��NH4+��SO42-��NO3- |
| ѡ�� | ʵ�� | ʵ��Ŀ�� |
| A | �ƺ�þ�ֱ�Ͷ����ˮ�� | �ж��ƺ�þ������ǿ�� |
| B | ��MgCl2��AlCl3��Һ�зֱ��������İ�ˮ | �ж�þ�����Ľ�����ǿ�� |
| C | ���������Һ��ͨ��CO2 | �ж�̼������������ǿ�� |
| D | Br2��I2�ֱ���������H2��Ӧ | �ж������ķǽ�����ǿ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | �� CH3��2CHCH2CH2CH3 | B�� | ��CH3CH2��2CHCH3 | C�� | ��CH3��2CHCH��CH3��2 | D�� | ��CH3��3CCH3 |
| A�� | ��ϵͳ��������������  ������Ϊ2��4-���һ�-6-�������� ������Ϊ2��4-���һ�-6-�������� | |
| B�� | �¹�ϩ ��  ������̼ԭ��һ����ͬһƽ���� ������̼ԭ��һ����ͬһƽ���� | |
| C�� | ȡ±�����������������Ƶ��Ҵ���Һ����һ��ʱ�����ȴ���ټ���ϡ�����ữ����������Һ��һ������������������ݳ�����ɫ�ж�±������±ԭ�ӵ����� | |
| D�� | ͨ�������£�1mol��  �ֱ���H2����Ũ��ˮ��ȫ��Ӧʱ�����ĵ�H2�� �ֱ���H2����Ũ��ˮ��ȫ��Ӧʱ�����ĵ�H2��Br2�����ʵ����ֱ��� 4mol��3mol |
 ��
��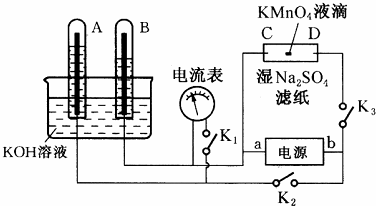
 ʵ������ȡ��ϩ��װ������ͼ��ʾ����ش�
ʵ������ȡ��ϩ��װ������ͼ��ʾ����ش�