��Ŀ����
6��±�أ�F��Cl��Br��I��At������������ҵ�г�����Ԫ�أ����û�ѧ����ش��������⣺��1��131I��һ�ֿ��Կ����䲢�й㷺ҽѧӦ�õ�ͬλ�أ���������Ϊ78��
��2������±�����ʣ�����˵����ȷ����ac��
a���õ���������Cl��Br��I b��IBr����Ϊ-1�ۣ���ˮ��Ӧ����HBrO��HI
c���е㣺Br2��Cl2 d����HIO3��HBrO3����������֪������Br2��I2
��3���������巨�ɴӺ�ˮ����ȡBr2����SO2������ˮ��ԭΪBr-��֮�����ÿ������������壬д��SO2��ԭ��ˮ�Ļ�ѧ����ʽSO2+Br2+2H2O=4H++SO42-+2Br-���ô������մ���Ҳ����Ϊ�����IJ���֮һ���������մ���ʱ��3molBr2���뷴Ӧ����ת��5mol���ӣ�д����Ӧ���ӷ���ʽ3Br2+3CO32-�TBr-+BrO3-+3CO2����
��4����NaClOŨ��Һ�м���KAl��SO4��2Ũ��Һ��Ѹ�����ɴ�����ɫ��״������ͬʱ����ɫ���������д����Ӧ�����ӷ���ʽΪ2Al3++6ClO-+6H2O=2Al��OH��3+6HCl��+2O2����
��5����ԭ��Cl-��Br-��I-��˳����������ԭ���ǵ��Ӳ������ӣ����Ӱ뾶����ʧ����������ǿ��
��6��CuBr2�ֽ���Ȼ�ѧ����ʽΪ��2CuBr2��s��?2CuBr��s��+Br2��g����H=+105.4kJ/mol
���ܱ������н�����CuBr2��487K�¼��ȷֽ⣬ƽ��ʱc��Br2��=0.1mol/L��p��Br2��Ϊ4.66��103Pa
�練Ӧ�¶Ȳ��䣬����Ӧ��ϵ���������һ������p��Br2���ı仯��ΧΪ��c��Br2��=2.33��103Pa��p��Br2����4.66��103Pa��
��7�������£���10mL1mol/LNaOH��Һ�м���20mL1mol/LHClO��Һ��������ҺpH=7.5
c��HClO��-c��ClO-��=1.8��10-6.5�������֣�
��8������������������
| ���� | �뾶 | ���ļ��� | �е� |
| H-Cl��H-I | Cl-��Br- | H-Cl��H-Br | HF��HI |
���� ��1��I��������Ϊ53�����������=������+���������㣻
��2��ͬ����Ԫ�ش��ϵ��£�Ԫ�صĽ���������ǿ���ǽ�����������
��3������������л�ԭ�ԣ�����������ԣ����߷���������ԭ��Ӧ����HBr�����
��4��NaClOŨ��Һ�м���KAl��SO4��2Ũ��Һ����������ˮ�ⷴӦ��
��5��ͬ����Ԫ�طǽ�����Խǿ����Ӧ�������ӻ�ԭ��Խǿ��
��6������Ӧ��ϵ���������һ����ѹǿ��С��ƽ�������ƶ���
��7����10mL1mol/LNaOH��Һ�м���20mL1mol/LHClO��Һ����Ӧ������NaClO��HClOʣ�࣬pH=7.5��˵��ClO-ˮ�����HClO����̶ȣ���ϵ���غ�������غ��жϣ�
��8��HF���������������Ӳ���Խ�࣬�뾶Խ�뾶Խ����ԽС���⻯���У�Ԫ�صķǽ�����Խǿ�����ļ���Խǿ��
��� �⣺��1��I��������Ϊ53����������=������+����������������Ϊ131-53=78���ʴ�Ϊ��78��
��2��a��ͬ����Ԫ�ش��ϵ��µõ�����������ǿ����a��ȷ��
b��IBr����Ϊ-1�ۣ���ˮ��Ӧ����HBr��HIO����b����
c����Ϊ���Ӿ��壬��Է�������Խ����е�Խ����е㣺Br2��Cl2����c��ȷ��
d����HIO3��HBrO3����������������ۺ����ᣬ�������ڱȽϷǽ����Ժ͵��ʵ������ԣ���d����
�ʴ�Ϊ��ac��
��3������������л�ԭ�ԣ�����������ԣ����߷���������ԭ��Ӧ����HBr�����ᣬ��Ӧ�����ӷ���ʽΪSO2+Br2+2H2O=4H++SO42-+2Br-���������մ���ʱ��3molBr2���뷴Ӧ����ת��5mol���ӣ���Ӧ����NaBrO3�����ӷ���ʽΪ3Br2+3CO32-�TBr-+BrO3-+3CO2����
�ʴ�Ϊ��SO2+Br2+2H2O=4H++SO42-+2Br-��3Br2+3CO32-�TBr-+BrO3-+3CO2����
��4��NaClOŨ��Һ�м���KAl��SO4��2Ũ��Һ����������ˮ�ⷴӦ�����ӷ���ʽΪ2Al3++6ClO-+6H2O=2Al��OH��3+6HCl��+2O2����
�ʴ�Ϊ��2Al3++6ClO-+6H2O=2Al��OH��3+6HCl��+2O2����
��5��ͬ����Ԫ�طǽ�����Խǿ����Ӧ�������ӻ�ԭ��Խǿ��ԭ���ǵ��Ӳ������ӣ����Ӱ뾶����ʧ����������ǿ���ʴ�Ϊ�����Ӳ������ӣ����Ӱ뾶����ʧ����������ǿ��
��6������Ӧ��ϵ���������һ����ѹǿ��С��ƽ�������ƶ���Ӧ����2.33��103Pa��p��Br2����4.66��103Pa���ʴ�Ϊ��2.33��103Pa��p��Br2����4.66��103Pa��
��7����10mL1mol/LNaOH��Һ�м���20mL1mol/LHClO��Һ����Ӧ������NaClO��HClOʣ�࣬pH=7.5��˵��ClO-ˮ�����HClO����̶ȣ���Һ�д���c��Na+��+c��H+��=c��ClO-��+c��OH-����c��HClO��+c��ClO-��=2c��Na+������c��HClO��-c��ClO-��=2c��OH-��-2c��H+��=2��10-6.5-2��10-7.5=1.8��10-6.5��
�ʴ�Ϊ��1.8��10-6.5��
��8��HF���������������Ӳ���Խ�࣬�뾶Խ�뾶Խ����ԽС���⻯���У�Ԫ�صķǽ�����Խǿ�����ļ���Խǿ������H-Cl��H-I���뾶Cl-��Br-�����ļ���
H-Cl��H-Br���е�HF��HI���ʴ�Ϊ������������������
���� ���⿼���Ϊ�ۺϣ��漰���֪ʶ���ۺϿ���ѧ���ķ��������ͼ���������Ϊ�߿��������ͺ�Ƶ���㣬ע�����֪ʶ�Ļ��ۣ��Ѷ��еȣ�

| A�� | Na2CO3 �Ǽ� | B�� | Na2C03 ���� | C�� | Na2C03������ | D�� | Na2CO3��̼���� |
| A�� | ʣ����������ͭ����� | |
| B�� | ��Ӧ����Һ��n��Fe3+��=0.10mol | |
| C�� | ԭ����������ͭ��������9.6g | |
| D�� | ��Ӧ����Һ��n��Fe2+��+n��Cu2+��=0.64mol |
| A�� | 18g 18O2 �к���NA����ԭ�� | |
| B�� | ��״���£�11.2 L���к��з��ӵ���ĿΪ0.5NA | |
| C�� | 17.6g�����������ļ��Թ��ۼ�Ϊ4NA�� | |
| D�� | �ڷ�ӦKIO3+6HI�TKI+3I2+3H2O�У�ÿ����3molI2ת�Ƶĵ�����Ϊ6NA |
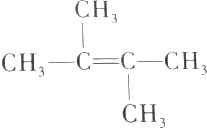 �������йظ�ϩ�����ӵ���������ȷ���ǣ�������
�������йظ�ϩ�����ӵ���������ȷ���ǣ�������| A�� | ��ϩ�������е�����ԭ�Ӷ���ͬһƽ���� | |
| B�� | ��ϩ����������4��̼ԭ�ӿ�����һ��ֱ���� | |
| C�� | ��ϩ�������е�6��̼ԭ�Ӳ����ܶ���ͬһƽ���� | |
| D�� | ��ϩ����CH2=C��CH2CH3��2��Ϊͬ���칹�� |
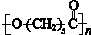 ����ϳ�·�����£�
����ϳ�·�����£�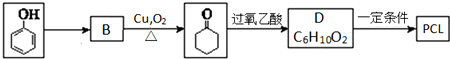

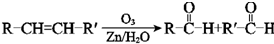
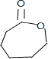 ����������D�ķ�Ӧ������������Ӧ��
����������D�ķ�Ӧ������������Ӧ��
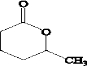 ��
�� ��XOHCCH2CH2CH2CH2CHO��
��XOHCCH2CH2CH2CH2CHO��