��Ŀ����
1��ͨ������£���������CO2�������������0.050%ʱ�����������Ե�����ЧӦ��Ϊ��С������CO2�Ի�����Ӱ�죬������������CO2��������ͬʱҲ��ǿ��CO2�������õ��о�����1��Ŀǰ���ƹ��ó��ٽ�CO2��������̬��Һ̬֮�䣩��������������������һ�����Ի����Ļ��������DZ��������㣮
��2����ѧ��Ϊ��ȡ�����е�CO2���ѿ�������̼�����Һ��Ȼ���ٰ�CO2����Һ����ȡ����������ѧ��Ӧʹ֮��Ϊ������ȼ�ϼ״���������ͼ��

�ٷֽ���з�Ӧ�Ļ�ѧ����ʽΪ��2KHCO3$\frac{\underline{\;\;��\;\;}}{\;}$K2CO3+H2O+CO2����
�ںϳ����У�����4.4g CO2������H2ǡ�÷�Ӧ������̬����ų�4.947kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ��CO2��g��+3H2��g���TCH3OH��g��+H2O��g����H=-49.47kJ/mol��
��3��ijͬѧ���ó������ⶨ������CO2����������������CaCO3��BaCO3���ܶȻ�Ksp�ֱ�Ϊ4.96��10-9��2.58��10-9������ý�����ͨ��������Ba��OH��2����NaOH��Һ��BaCl2��Һ����Һ��ʵ��ʱ���ⶨ�¶ȡ�ѹǿ�Ϳ���������⣬����ⶨʵ��ʱ���¶ȡ�ѹǿ��������������
���� ��1���������ܲ�����ԭ�ӣ�����ԭ���dz����ֽ�Ĵ�����
��2���ٸ���̼��������ȷֽ�Ļ�ѧ���ʻش�
�ںϳ����з�����ӦΪ������̼��������Ӧ���ɼ״���ˮ������4.4 kg CO2������H2ǡ����ȫ��Ӧ���ɷų�4.947kJ����������1 mol CO2�������ϳɼ״��ų�����49.47 kJ����������д�Ȼ�ѧ��Ӧ����ʽʱ��Ӧע������״̬����Ӧ�ȵ��������Լ���λ��
��3��CaCO3��BaCO3���ܶȻ���С��֪BaCO3�����ܣ������CO2����BaCO3��Ӧ����ȫ��
��� �⣺��1���������ڿ������ܲ�����ԭ�ӣ�����ԭ���dz����ֽ�Ĵ�����CO2��������ɷ�ֹ�������ƻ����ʴ�Ϊ�����������㣻
��2����̼��������ȿɷֽ��̼��ء�������̼��ˮ������ʽΪ��2KHCO3$\frac{\underline{\;\;��\;\;}}{\;}$K2CO3+H2O+CO2�����ʴ�Ϊ��2KHCO3$\frac{\underline{\;\;��\;\;}}{\;}$K2CO3+H2O+CO2����
�ڸ���4.4 kg CO2������H2ǡ����ȫ��Ӧ���ɷų�4.947 kJ����������1 mol CO2�������ϳɼ״��ų�����49.47 kJ�������������Ȼ�ѧ����ʽΪCO2��g��+3H2��g���TCH3OH��g��+H2O��g����H=-49.47kJ/mol���ʴ�Ϊ��CO2��g��+3H2��g���TCH3OH��g��+H2O��g����H=-49.47kJ/mol��
��3������CaCO3��BaCO3���ܶȻ���С��֪BaCO3�����ܣ������CO2����BaCO3��Ӧ����ȫ���ʿ�ѡ��Ba��OH��2����NaOH��Һ��BaCl2��Һ����ΪCO2�ij��������ⶨ�����ݳ�����������⣬����Ҫ�ⶨʵ��ʱ���¶ȡ�ѹǿ���������������ʴ�Ϊ��Ba��OH��2����NaOH��Һ��BaCl2��Һ����ʵ��ʱ���¶ȡ�ѹǿ��������������
���� �����Զ�CO2�������õ��о�Ϊ���壬�����Ȼ�ѧ����ʽ��������ѡ���֪ʶ���Ѷ��еȣ�ּ�ڿ���ѧ����֪ʶ�����ռ�Ǩ������������

 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�| A�� | �Խ�̿�Ͷ�������Ϊԭ���������ʹ� | |
| B�� | ����Ϊԭ���������� | |
| C�� | ����Ȼ�þ��Һ��������þ | |
| D�� | ��������Ȼ������������� |
| A�� | �û���ˮ��Һ�ʼ��� | |
| B�� | �û�����H2SO4��Ӧ���������� | |
| C�� | �û�����Һ����BaCl2�������ɰ�ɫ���� | |
| D�� | �û��������������¿�����S��ÿ����32g Sת��2NA������ |
 �������£���0.02mol/L��Na2CrO4��Һ�еμ�0.01mol/L��ϡ���ᣬ��Һ�ɻ�ɫת��ɳȺ�ɫ��Na2Cr2O7����Һ��ˮKw�����ڴ�ת�������У������ʵ���Ũ�ȱ仯��ͼ��ʾ������˵����ȷ���ǣ�������
�������£���0.02mol/L��Na2CrO4��Һ�еμ�0.01mol/L��ϡ���ᣬ��Һ�ɻ�ɫת��ɳȺ�ɫ��Na2Cr2O7����Һ��ˮKw�����ڴ�ת�������У������ʵ���Ũ�ȱ仯��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | �����Ϸ�Ӧ����һ������Na2CO3��Һ����ƽ�⼸��û��Ӱ�� | |
| B�� | �����¶ȣ������Һ����ɫ���� | |
| C�� | �÷�Ӧ�����ӷ���ʽΪ��2CrO42-+2H+�TCr2O72-+H2O | |
| D�� | �÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ5.0x102 |
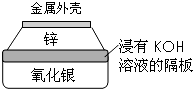 ������Ϊһ����Դ�ܵ�Խ��Խ��Ĺ�ע��
������Ϊһ����Դ�ܵ�Խ��Խ��Ĺ�ע��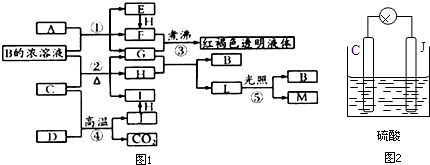
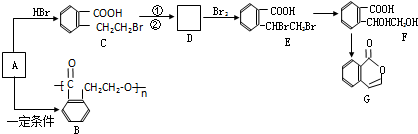
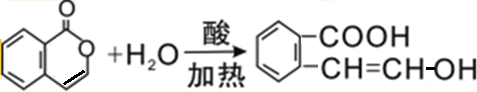
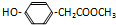 ��
��