��Ŀ����
����Ŀ��AA705�Ͻ�(��Al��Zn��Mg��Cu)�������һ����̣���������Ϊ�ֵ�����֮һ���ѱ����ڷɻ������ͻ����������ֻ�����ϵȡ������ֺϽ���ѱ����ӡ������ѧ�ҽ�̼����������(��С��Ϊʮ�ڷ�֮һ��)ע��AA7075�ĺ�˿�ڣ�����Щ�������䵱���Ӽ�֮��������ϡ�ע�����������ӵ���亸˿Ҳ���Ը����������������Ժ��ӵĽ����ͽ����Ͻ𡣻ش��������⣺
(1)��̬ͭԭ�ӵļ۲�����Ų�ʽΪ__________��
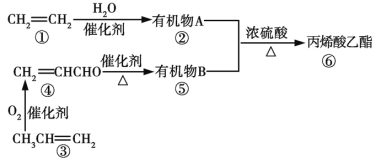
(2)��������ijԪ�ص�ǰ5�����ӵĵ�������ͼ1��ʾ����Ԫ����_____(��Ԫ�ط���)���ж�������_______��
(3)CN����NH3��H2O��OH�������嶼����Zn2+�γ������ӡ�1mol [Zn(NH3)4]2+��___ mol�������������ӵ���λ��Ϊ_____��
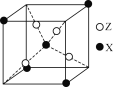
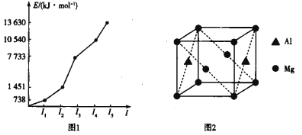
(4)��þ�Ͻ������ʴ��Ʋ���,ԭ��λ�����ĺͶ��㣬�侧����ͼ2��ʾ��1����ԭ����Χ��_____��þԭ������ҵȾ��롣
(5)�ڶ������Ѻ��������£����״��ɱ������ɱ���ȩ��

�ٱ��״���Cԭ���ӻ�������__________��
�ڱ��״��ķе���ڱ���ȩ����ԭ����__________��
(6)�Ѿ���������Ʒ������ͼ��ʾ��
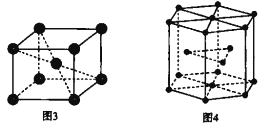
����ͼ3��ʾ�������Ŀռ�������Ϊ______(�ú�����ʽ�ӱ�ʾ)��
����֪ͼ4���������߳�Ϊx cm����Ϊy cm�����Ѿ����ܶ�ΪD g��cm-3��NAΪ______mol��1(�ú�x y��D��ʽ�ӱ�ʾ)��
���𰸡�3d104s1 Mg I3��I2��5���࣬˵���������2�����ӣ� 16 4 8 sp2��sp3 ���״����Ӽ�������������ȩ���Ӽ䲻������� ![]()
![]()
��������
��1��ͭԭ����29�����ӣ���3d��4s�ܼ��ϵĵ���Ϊ��۵��ӣ�
(2)���ݵ����ܵ�ͻ���ж��������ӣ���ϸ�Ԫ��Ϊ��������Ԫ�ط�����
(3)���е���������λ����Ϊ������˫������һ��Ϊ������ÿ��NH3�����к���3��N-H��������ԭ��Zn���ĸ�Nԭ��֮�������λ����
(4)������ÿ�������4�������ϵ�þԭ�Ӻ������ϵ�þԭ�ӵ�Alԭ�ӵľ�������������
(5)�ٱ�����̼ԭ���γ�3�����õ��Ӷԣ���-CH2OH��Cԭ���γ�4�����õ��Ӷԣ�
�ڷ��Ӽ����������ʵķе�Ӱ��ϴ��״����Ӽ���������
(6)����ͼ3��֪����������ԭ�ӵ���ĿΪ1+8��![]() =2����ԭ�Ӱ뾶Ϊr�����ĶԽ���Ϊ4r�������ı߳�Ϊ
=2����ԭ�Ӱ뾶Ϊr�����ĶԽ���Ϊ4r�������ı߳�Ϊ![]() ���ɴ˼���ռ������ʣ�
���ɴ˼���ռ������ʣ�
��ͼ4��������ԭ�ӵ���ĿΪ3+2��![]() +12��
+12��![]() =6������������Ϊ
=6������������Ϊ![]() g���������߳�Ϊx cm����Ϊy cm���������Ϊ
g���������߳�Ϊx cm����Ϊy cm���������Ϊ![]() x2ycm3���ٽ�Ͼ������ܶȼ���NA��
x2ycm3���ٽ�Ͼ������ܶȼ���NA��
��1��ͭԭ����29�����ӣ���3d��4s�ܼ��ϵĵ���Ϊ��۵��ӣ���۵����Ų�ʽΪ3d104s1��
(2)��ͼ1��֪������I3��I2��5���࣬˵���������2�����ӣ���ϸ�Ԫ���ǵ�������Ԫ�أ����Ԫ��Ϊ�������ڵڢ�AԪ�أ���Ԫ��Ϊþ��Ԫ�ط���ΪMg��
(3)���е���������λ����Ϊ������˫������һ��Ϊ������ÿ��NH3�����к���3��N-H��������ԭ��Zn���ĸ�Nԭ��֮�������λ������1mol [Zn(NH3)4]2+����4+3��4��mol=16mol��������λ��ΪNH3����������Zn2+����λ��Ϊ4��
(4)������ÿ�������4�������ϵ�þԭ�Ӻ������ϵ�þԭ�ӵ�Alԭ�ӵľ���������������ÿ����ԭ����Χ���������þԭ����8����
(5)�ٱ�����̼ԭ���γ�3�����õ��Ӷԣ�̼ԭ�ӵ��ӻ�������sp2����-CH2OH��Cԭ���γ�4�����õ��Ӷԣ�̼ԭ�ӵ��ӻ�������sp3��
�ڱ��״����Ӽ���������������ȩ���Ӽ䲻������������±��״��ķе����Աȱ���ȩ�ߣ�
(6)����ͼ3��֪����������ԭ�ӵ���ĿΪ1+8��![]() =2����ԭ�Ӱ뾶Ϊr�����ĶԽ���Ϊ4r�������ı߳�Ϊ
=2����ԭ�Ӱ뾶Ϊr�����ĶԽ���Ϊ4r�������ı߳�Ϊ![]() ����ռ�������Ϊ
����ռ�������Ϊ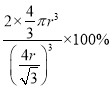 =
=![]() ��
��
�ڢ�ͼ4��������ԭ�ӵ���ĿΪ3+2��![]() +12��
+12��![]() =6������������Ϊ
=6������������Ϊ![]() g���������߳�Ϊx cm����Ϊy cm���������Ϊ
g���������߳�Ϊx cm����Ϊy cm���������Ϊ![]() x2ycm3����D=
x2ycm3����D=![]() g��
g��![]() x2ycm3���ɴ˼����NA=
x2ycm3���ɴ˼����NA=![]() mol��1��
mol��1��

����Ŀ��ij��ѧ����С���ͬѧͨ��ʵ��̽����ʶ��ѧ��Ӧ���ʺͻ�ѧ��Ӧ�ȡ�
��1��ʵ��һ��̽���¶Ⱥ�Ũ�ȶԷ�Ӧ���ʵ�Ӱ��
ʵ��ԭ������������������Һ�У������(KIO3)���������ƿɷ�����Ӧ���ɵ⣬��Ӧԭ����2IO3-��5SO32-��2H��===I2��5SO42-��H2O�����ɵĵ���õ�����Һ���飬���ݳ�����ɫ�����ʱ���������÷�Ӧ�����ʡ�
ʵ����� | 0.01 mol��L��1 KIO3������Һ(������)�����/mL | 0.01 mol��L��1 Na2SO3��Һ�����/mL | ˮ�����/mL | ʵ���¶�/�� | ������ɫ��ʱ��/s |
�� | 5 | 5 | V1 | 0 | |
�� | 5 | 5 | 40 | 25 | |
�� | 5 | V2 | 35 | 25 |
��V1��________ mL��V2��________ mL��
��2��ʵ�����̽��KI��FeCl3���ʱ����KCl��I2��FeCl2�ķ�Ӧ����һ�����ȡ�ʵ�鲽�裺
��.��5 mL 0.1 mol��L��1 KI��Һ�еμ�5��6��0.1 mol��L��1 FeCl3��Һ����ַ�Ӧ��������Һ�ֳɼס��ҡ������ȷݣ�
��.����еμ�CCl4�������
��.�����еμ��Լ�X��
�ٽ�KI��FeCl3��Ӧ�����ӷ���ʽ����������____I����____Fe3�� ![]() ____I2��____Fe2����
____I2��____Fe2����
�ڲ��袣�У��Լ�X��_________________��
�۲��袢�͢��е�ʵ������˵��KI��FeCl3���ʱ����KCl��I2��FeCl2�ķ�Ӧ����һ�����ȣ���ʵ��������________________________________��