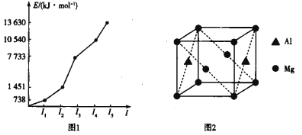
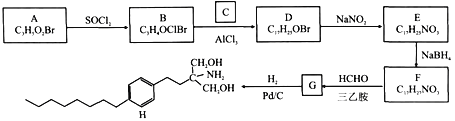
��Ŀ����
����Ŀ��ij��ɫ����Һ�п��ܺ������������еļ��֣�Na����K����NH4����Ag����Mg2����Cu2����Cl����CO32����SO42���������ĸ��������ʵ���Ũ����ȣ�ȡ���ݽ�������ʵ�飺
�����һ���м�������BaCl2��Һ���õ���ɫ���������˺�������ϴ�ӳ��������������ʧ
����ڶ��ݼ������������ữ��AgNO3��Һ����Һ���ְ�ɫ����
��������ݼ�������NaOH��Һ�����ȣ��ռ���һ����������壬����Һ��Ȼ����
�Իش��������⣺
(1)�϶����ڵ�������______________________
(2)д�����з��������ӷ�Ӧ����ʽ________________��___________________
(3)д�����з��������ӷ�Ӧ����ʽ________________��___________________
(4)д�����з��������ӷ�Ӧ����ʽ_________________________
���𰸡�Na����K����NH4����Cl����CO32�� Ba2����CO32����BaCO3���� BaCO3��2H����Ba2����CO2�� Ag����Cl����AgCl���� 2H����CO32����H2O��CO2�� NH4����OH��![]() NH3����H2O
NH3����H2O
��������
��ɫ����Һ��һ������Cu2���������һ���м�������BaCl2��Һ���õ���ɫ���������˺�������ϴ�ӳ��������������ʧ��˵���õ���ɫ������BaCO3������BaSO4��AgCl������ԭ��Һһ������CO32����һ������Ag����SO42����������CO32��������һ������Mg2��������ڶ��ݼ������������ữ��AgNO3��Һ����Һ���ְ�ɫ������˵��һ������Cl������������ݼ�������NaOH��Һ�����ȣ��ռ���һ����������壬����Һ��Ȼ���壬˵��ԭ��Һһ������NH4�������ݵ�����ƶ�Na����K����
�������Ϸ����� (1)��Һ��NH4����Cl����CO32����û��Ag����Mg2����Cu2����SO42���������ĸ��������ʵ���Ũ����ȣ����ݵ���أ���Һ����Na����K�����϶����ڵ�������Na����K����NH4����Cl����CO32����
(2)����CO32����Ba2������BaCO3��������Ӧ�����ӷ���ʽ��Ba2����CO32����BaCO3����̼�ᱵ�����ᷴӦ�����Ȼ�����������̼��ˮ����Ӧ�����ӷ���ʽ��BaCO3��2H����Ba2����CO2����
(3)�ڼ������ᣬ�����̼������ӷ�Ӧ���ɶ�����̼��ˮ����Ӧ�����ӷ���ʽ��2H����CO32����H2O��CO2����Ag����Cl������AgCl������������Ӧ�����ӷ���ʽ��Ag����Cl����AgCl����
(4)�������������ӡ�笠����ӷ�Ӧ���ɰ�������Ӧ�����ӷ�Ӧ����ʽ��NH4����OH��![]() NH3����H2O��
NH3����H2O��

����Ŀ��FeCl2��һ�ֳ��õĻ�ԭ�����й��������£�
C6H5Cl(�ȱ�) | C6H4Cl2 | FeCl3 | FeCl2 | |
�ܽ��� | ������ˮ�������ڱ� | ������C6H5Cl��C6H4Cl2����������ˮ | ||
�۵�/�� | ��45 | 53 | �� | �� |
�е�/�� | 132 | 173 | �� | �� |
ʵ���ҿ����ö��ַ������Ʊ���ˮFeCl2���ش��������⣺
��.������ͼװ����H2��ԭ��ˮFeCl3��ȡ��
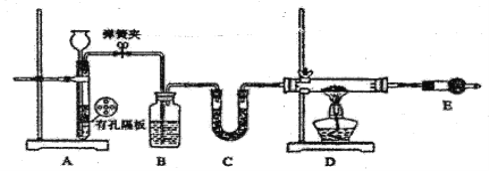
��1����װ��A��ȡH2�����ŵ���________________��D�з�Ӧ�Ļ�ѧ����ʽΪ____________________��װ��E��������____________________________��
��2��ͨ������H2����ַ�Ӧ������¶ȿ��Ʋ�������Ʒ�лẬ�������������Ʒ���Ƿ����ķ�����____________________________��
��.��ͼװ�ã���������ƿ�з���162.5g��ˮ�Ȼ�����225g�ȱ������Ʒ�Ӧ�¶���128�桫139�����3h����Ӧ�ӽ�100%����Ӧ���£�2FeCl3+C6H5Cl��2FeCl2+C6H4Cl2+HCl
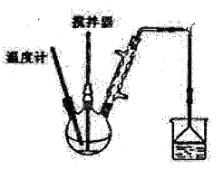
��3��������Ӧ�У���ԭ����____________��
��4����Ӧ�¶Ƚӽ���C6H5Cl�ķе㣬��ʵ�������C6H5Cl�����������ʧ��ԭ����____________________________��
��5����ȴ������ƿ�����ʾ������ˣ�ϴ�ӣ�����õ��ֲ�Ʒ��
��ϴ�����õ��Լ�������____________________________��
�ڼ���������Һ��C6H5Cl�ķ���____________________________��