��Ŀ����
��12�֣�����ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֡�K2Cr2O7��CrO3�����������ӡȾ�����ϡ���Ƶȹ�ҵ�У��ǹ�ҵ����ɸ���Ⱦ����Ҫԭ���ڱ���ġ������ҡ��¼��У�������Ϊ�ù�ҵƤ����½��ϻ���ƤЬ��Ϊԭ���ƳɵĹ�ҵ������ð���ʳ�������Ƴɽ��ң���ɽ����ڵĸ����س��ꡣ
��1��CrO3�����ȶ��Խϲ����ʱ�ֽ⣬�������������¶ȵı仯��ͼ��ʾ��
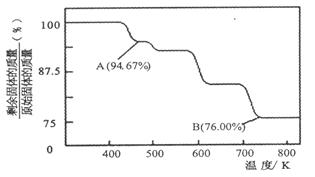
��A ��ʱʣ�����ijɷ��� ���ѧʽ����
�ڴӿ�ʼ���ȵ� 750K ʱ�ܷ�Ӧ����ʽΪ ��
��2��Cr(��)��Ҫ��CrO42����Cr2O72����̬���ڣ������������¾��к�ǿ�������ԣ���������Һ�д�������ת����CrO42��(��ɫ)+2H+ Cr2O72��(��ɫ��+H2O��K=4.2��1014����Ҫʹ��Һ�ɻ�ɫ���ɫ����Ӧ��ȡ�Ĵ�ʩ�� ��
Cr2O72��(��ɫ��+H2O��K=4.2��1014����Ҫʹ��Һ�ɻ�ɫ���ɫ����Ӧ��ȡ�Ĵ�ʩ�� ��
��3����ҵ��ˮ�г�����һ������Cr(��)�����Խϴ����ǻ�����༰��̬ϵͳ�����ܴ���������������֮һ�ǽ���Cr2O72���ķ�ˮ��������ڣ�����������������������NaCl���е�⣺���������ɵ�Fe2����Cr2O72��������Ӧ�����ɵ�Fe3����Cr3������������OH���������Fe(OH)3��Cr(OH)3�����Գ�ȥ[��֪KspFe(OH)3��4.0��10��38��KspCr(OH)3��6.0��10��31]��
�ٵ������� NaCl �������� ��
��д�������ĵ缫��Ӧʽ ��
��д��Fe2����Cr2O72��������Ӧ����Fe3����Cr3�������ӷ�Ӧ����ʽ ��
����֪�������Һ��c(Fe3��)=2.0��10��13 mol��L��1������Һ��c(Cr3��)Ϊ mol��L��1��
��1��CrO3�����ȶ��Խϲ����ʱ�ֽ⣬�������������¶ȵı仯��ͼ��ʾ��

��A ��ʱʣ�����ijɷ��� ���ѧʽ����
�ڴӿ�ʼ���ȵ� 750K ʱ�ܷ�Ӧ����ʽΪ ��
��2��Cr(��)��Ҫ��CrO42����Cr2O72����̬���ڣ������������¾��к�ǿ�������ԣ���������Һ�д�������ת����CrO42��(��ɫ)+2H+
 Cr2O72��(��ɫ��+H2O��K=4.2��1014����Ҫʹ��Һ�ɻ�ɫ���ɫ����Ӧ��ȡ�Ĵ�ʩ�� ��
Cr2O72��(��ɫ��+H2O��K=4.2��1014����Ҫʹ��Һ�ɻ�ɫ���ɫ����Ӧ��ȡ�Ĵ�ʩ�� ��| A����NaOH | B�������� | C�������� | D����AgNO3 |
�ٵ������� NaCl �������� ��
��д�������ĵ缫��Ӧʽ ��
��д��Fe2����Cr2O72��������Ӧ����Fe3����Cr3�������ӷ�Ӧ����ʽ ��
����֪�������Һ��c(Fe3��)=2.0��10��13 mol��L��1������Һ��c(Cr3��)Ϊ mol��L��1��
��1����Cr3O8����CrO2.67) ��2�֣� �� 4CrO32Cr2O3��3O2����2�֣�
��2��C ��2�֣� (3) ����ǿ��Һ�ĵ����� ��1�֣� ��Fe-2e��=Fe2+ ��1�֣�
��6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O ��2�֣���3.0��10��6 ��2�֣�
��2��C ��2�֣� (3) ����ǿ��Һ�ĵ����� ��1�֣� ��Fe-2e��=Fe2+ ��1�֣�
��6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O ��2�֣���3.0��10��6 ��2�֣�
��1���ټ��������100g�����ʵ�����1mol��������ԭ����3mol��A�������94.67g�����ٵ�������5.33g�������ٵ���ԭ����0.333mol�����Բ���Cr����ԭ�ӵ����ʵ���֮����1�U��3��1/3��=3�U8,���Ի�ѧʽΪCr3O8��
�ڼ��ȵ� 750K ʱ��ʣ�����������76.0g�����ݢټ���ԭ��ͬ�����Եó���ʱ������Cr2O3��ʣ���ʽΪ4CrO32Cr2O3��3O2����
��2��Ҫʹ��Һ�ɻ�ɫ���ɫ����ƽ��Ӧ��������Ӧ�����ƶ�����������������Ũ�ȿ��ԣ�A����ȷ������Ũ�����ܱ�����������ѡ��B��C��ȷ��D�л����ɸ��������������淴Ӧ�����ƶ�������ȷ����ѡC��
��3��������ˮ�ĵ��������ϲ���Ȼ�����ǿ����ʣ�����ǿ��Һ�ĵ����ԡ�
������ʧȥ���ӣ��������������Է���ʽΪFe-2e��=Fe2+��
�۸��ݷ�Ӧ����������֪������ʽΪ6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O��
�ܸ��ݶ��ߵ��ܶȻ���������ʽ��֪����Һ��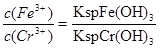 �����ڵ������Һ��c(Fe3+)Ϊ2.0��10��13 mol��L��1������Һ��c(Cr3+)Ϊ
�����ڵ������Һ��c(Fe3+)Ϊ2.0��10��13 mol��L��1������Һ��c(Cr3+)Ϊ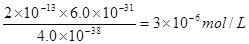 ��
��
�ڼ��ȵ� 750K ʱ��ʣ�����������76.0g�����ݢټ���ԭ��ͬ�����Եó���ʱ������Cr2O3��ʣ���ʽΪ4CrO32Cr2O3��3O2����
��2��Ҫʹ��Һ�ɻ�ɫ���ɫ����ƽ��Ӧ��������Ӧ�����ƶ�����������������Ũ�ȿ��ԣ�A����ȷ������Ũ�����ܱ�����������ѡ��B��C��ȷ��D�л����ɸ��������������淴Ӧ�����ƶ�������ȷ����ѡC��
��3��������ˮ�ĵ��������ϲ���Ȼ�����ǿ����ʣ�����ǿ��Һ�ĵ����ԡ�
������ʧȥ���ӣ��������������Է���ʽΪFe-2e��=Fe2+��
�۸��ݷ�Ӧ����������֪������ʽΪ6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O��
�ܸ��ݶ��ߵ��ܶȻ���������ʽ��֪����Һ��
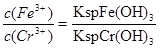 �����ڵ������Һ��c(Fe3+)Ϊ2.0��10��13 mol��L��1������Һ��c(Cr3+)Ϊ
�����ڵ������Һ��c(Fe3+)Ϊ2.0��10��13 mol��L��1������Һ��c(Cr3+)Ϊ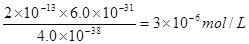 ��
��
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
