��Ŀ����
��12�֣�����������һ��ʹ�ù㷺�Ļ�ѧ�Լ���ij����С��ͨ������ʵ��ⶨij������Ba(OH)2��nH2O�ĺ�����
��1����ȡ3.50 g������������ˮ���100 mL��Һ������ȡ��10.0 mL��Һ����ƿ�У���0.100 mol / L HCl����Һ��Ӧ����ȫ��Ӧ�����ı�Һ20.0 mL�����ʲ����ᷴӦ�������������������������ʵ�����
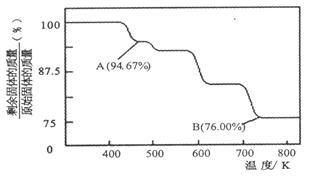
��2����ȡ5.25 g����������ʧȥȫ���ᾧˮ�����ʲ��ֽ⣩���Ƶ�����Ϊ3.09 g����Ba(OH)2��nH2O�е�nֵ��
��3��������Ba(OH)2��nH2O����������Ϊ
��1����ȡ3.50 g������������ˮ���100 mL��Һ������ȡ��10.0 mL��Һ����ƿ�У���0.100 mol / L HCl����Һ��Ӧ����ȫ��Ӧ�����ı�Һ20.0 mL�����ʲ����ᷴӦ�������������������������ʵ�����
��2����ȡ5.25 g����������ʧȥȫ���ᾧˮ�����ʲ��ֽ⣩���Ƶ�����Ϊ3.09 g����Ba(OH)2��nH2O�е�nֵ��
��3��������Ba(OH)2��nH2O����������Ϊ
��1��0.01 mol ��2��8 ��3��0.90
��1��0.01 mol
��2����Ʒ����Ϊ��1����1.5��
H2O�����ʵ���Ϊ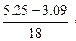 mol��0.12 mol��n��
mol��0.12 mol��n��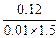 ��8
��8
��3��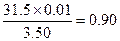
��2����Ʒ����Ϊ��1����1.5��
H2O�����ʵ���Ϊ
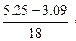 mol��0.12 mol��n��
mol��0.12 mol��n��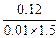 ��8
��8��3��
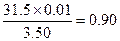

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 Cr2O72��(��ɫ��+H2O��K=4.2��1014����Ҫʹ��Һ�ɻ�ɫ���ɫ����Ӧ��ȡ�Ĵ�ʩ�� ��
Cr2O72��(��ɫ��+H2O��K=4.2��1014����Ҫʹ��Һ�ɻ�ɫ���ɫ����Ӧ��ȡ�Ĵ�ʩ�� ��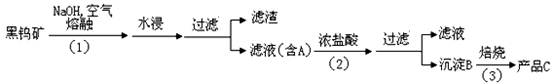
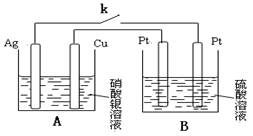
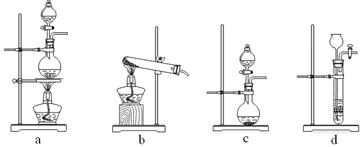
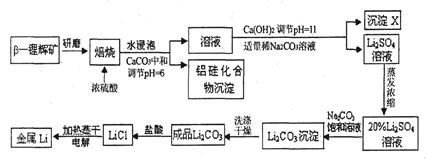
 a2CO3��Һ����˵��ǰ��Ũ�Ȳ�ͬ��ԭ��:
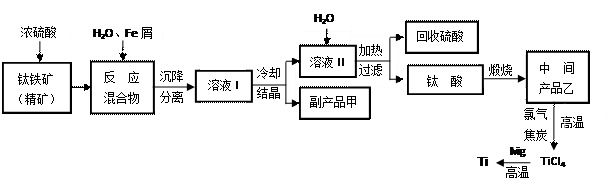
a2CO3��Һ����˵��ǰ��Ũ�Ȳ�ͬ��ԭ��: