��Ŀ����
1��һ�ְ�ɫ����A��������ˮ����A�����Һ�������ͼ��ʾ��ʵ�飬ʵ������ת����ϵ���ͼ��ʾ��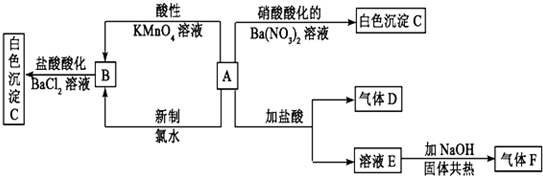
����AΪ���Σ�����D��ʹƷ����Һ��ɫ������F��ʹʪ��ĺ�ɫʯ����ֽ�������Իش��������⣺
��1��д���������ʻ�ѧʽ��A����NH4��2SO3��C��BaSO4��
��2��д�����з�Ӧ�����ӷ���ʽ��
����ҺE���������ƹ��ȣ�NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O
��A������KMnO4��Һ��Ӧ��2MmO4-+6H++5SO32-=5SO42-+2Mn2++5H2O��
���� ��ʹƷ����Һ��ɫ���Ƕ�����������D�Ƕ���������ʹʪ��ĺ�ɫʯ����ֽ�������ǰ�������F�ǰ�����A��һ�ְ�ɫ���壬���������ʷ�Ӧ�������ɰ����������ɶ�����������A�ǣ�NH4��2SO3�����ױ�����������Ϊ����泥�����B������泥��ó�E���Ȼ�泥���ɫ����C�����ᱵ��������ʵ����ʿɽ����⣮
��� �⣺��ʹƷ����Һ��ɫ���Ƕ�����������D�Ƕ���������ʹʪ��ĺ�ɫʯ����ֽ�������ǰ�������F�ǰ�����A��һ�ְ�ɫ���壬���������ʷ�Ӧ�������ɰ����������ɶ�����������A�ǣ�NH4��2SO3�����ױ�����������Ϊ����泥�����B������泥��ó�E���Ȼ�泥���ɫ����C�����ᱵ��
��1���ܺ��������ʷ�Ӧ�������ɰ����������ɶ�������İ�ɫ�����ǣ�NH4��2SO3������狀��Ȼ����������ֽⷴӦ�������ᱵ����������C��BaSO4��
�ʴ�Ϊ����NH4��2SO3��BaSO4��
��2������κ�ǿ��Ȼ����ɰ�������һ�ּ������壬ԭ��ΪNH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O��
�ʴ�Ϊ��NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O��
��A�ǣ�NH4��2SO3��A������KMnO4��Һ��Ӧ�����ӷ���ʽΪ2MmO4-+6H++5SO32-=5SO42-+2Mn2++5H2O��
�ʴ�Ϊ��2MmO4-+6H++5SO32-=5SO42-+2Mn2++5H2O��
���� ������һ����ͼ�ƶ��⣬ע��Ѱ�ҽ����ͻ�ƿ��ǹؼ�����Ҫ�������ʵ����ʣ��ѶȲ������ʱע��������ӵļ��鷽����ʵ����ƣ�

| A�� | ���ˡ�����������Һ | B�� | ���ˡ�������������Һ | ||
| C�� | �����������ˡ���Һ | D�� | ��Һ���������������� |
| A�� | 1mol��ϩ�����к��еĹ��ۼ���ĿΪ4NA | |
| B�� | 2L 0.5mol•L-1��NH4��2SO4��Һ��NH4+��������Ϊ2NA | |
| C�� | 1mol FeBr2������������Ӧʱ��ת�Ƶĵ�����Ϊ3NA | |
| D�� | 1mol Na2O2�����к���������Ϊ4NA |
 �������йص����ص�˵��������ǣ�������
�������йص����ص�˵��������ǣ�������| A�� | ��ȼ������ȫȼ������CO2��H2O | B�� | ���ܷ����ӳɷ�Ӧ | ||
| C�� | ���Է���������Ӧ | D�� | ����NaHCO3��Na��Ӧ |
| A�� | ��Fe��OH��3�����������Fe��OH��3+3H+�TFe3++3H20 | |
| B�� | ��H2S����ͨ�뵽CuS04��Һ�У�S2-+Cu2+�TCuS�� | |
| C�� | ��l mol FeBr2����Һ��ͨ��l mol Cl2��2Fe2++2Br-+2Cl2�T2Fe3++Br2+4Cl- | |
| D�� | ��Al2��S04��3��Һ�м������Ba��OH��2��Һ���а�ɫ��������2Al3++3S042-+3Ba2++60H-�T2AI��OH��3��+3BaS04�� |
| A�� | ����${\;}_{1}^{2}$H����������0 | |
| B�� | 12C��14C��Ϊͬλ�� | |
| C�� | ���ʯ��ʯī����ϩ��Ϊͬ�������� | |
| D�� | CH3CH2OH��CH3OCH3��Ϊͬ���칹�� |
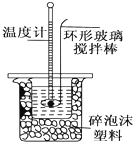 ijʵ��С����0.50mol•L-1 NaOH��Һ��0.50mol•L-1������Һ�����к��ȵIJⶨ��
ijʵ��С����0.50mol•L-1 NaOH��Һ��0.50mol•L-1������Һ�����к��ȵIJⶨ��������0.50mol•L-1 NaOH��Һ����ʵ���д�ԼҪʹ��245mL NaOH��Һ��������Ҫ����NaOH����0.50mol•L-1��0.25L��40g•mol-1=5.0 g��������ʽ��������������ʽ����������֣�
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ���к���Ϊ57.3kJ•mol-1����_$\frac{1}{2}$H2SO4��aq��+NaOH��aq���T$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ•mol-1_��
��2��ȡ50mL NaOH��Һ��30mL������Һ����ʵ�飬ʵ���������±���
������д�±��еĿհף�
| �¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ��t2-t1���� | ||
| H2SO | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 31.2 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
������ʵ�����ݽ����57.3kJ•mol-1��ƫ�����ƫ���ԭ������ǣ�����ĸ��a��b��c��d��
a��ʵ��װ�ñ��¡�����Ч����
b������NaOH��Һ����ʱ���ӿ̶�
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ�
| A�� | NH3 | B�� | HF | C�� | C2H5OH | D�� | CH4 |