��Ŀ����
10��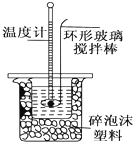 ijʵ��С����0.50mol•L-1 NaOH��Һ��0.50mol•L-1������Һ�����к��ȵIJⶨ��
ijʵ��С����0.50mol•L-1 NaOH��Һ��0.50mol•L-1������Һ�����к��ȵIJⶨ��������0.50mol•L-1 NaOH��Һ����ʵ���д�ԼҪʹ��245mL NaOH��Һ��������Ҫ����NaOH����0.50mol•L-1��0.25L��40g•mol-1=5.0 g��������ʽ��������������ʽ����������֣�
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ���к���Ϊ57.3kJ•mol-1����_$\frac{1}{2}$H2SO4��aq��+NaOH��aq���T$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ•mol-1_��
��2��ȡ50mL NaOH��Һ��30mL������Һ����ʵ�飬ʵ���������±���
������д�±��еĿհף�
| �¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ��t2-t1���� | ||
| H2SO | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 31.2 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
������ʵ�����ݽ����57.3kJ•mol-1��ƫ�����ƫ���ԭ������ǣ�����ĸ��a��b��c��d��
a��ʵ��װ�ñ��¡�����Ч����
b������NaOH��Һ����ʱ���ӿ̶�
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ�
���� ��1�����ݹ�ʽm=nM=cVM�������������Ƶ�����������û��245mL������ƿ��
��1���ٸ����к�����ǿ���ǿ���ϡ��Һ��ȫ��Ӧ����1molˮ�ų�����������ע���ʾۼ�״̬�Ͷ�Ӧ�ʱ�д���Ȼ�ѧ����ʽ��
��2�������ж��¶Ȳ����Ч�ԣ�Ȼ������¶Ȳ�ƽ��ֵ��
���ȸ���Q=m•c•��T���㷴Ӧ�ų���������Ȼ����ݡ�H=-$\frac{Q}{n}$kJ/mol�������Ӧ�ȣ�
��a��װ�ñ��¡�����Ч�����õ�����ƫС��
b������NaOH��Һ����ʱ���ӿ̶ȣ���Һ�����ƫ��Ũ��ƫС��
c����ΰ�NaOH��Һ����ʢ�������С�ձ��У�����ɢʧ����õ�����ƫС��
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ��������ʼ�¶�ƫ�ߣ�
��� �⣺����Ҫ����NaOH����m=nM=cVM=0.50 mol•L-1��0.25L��40g•mol-1=5.0g��
�ʴ�Ϊ��0.50 mol•L-1��0.25L��40g•mol-1=5.0��
��1���к�����ǿ���ǿ���ϡ��Һ��ȫ��Ӧ����1molˮ�ų���������ǿ��ǿ����к���Ϊ57.3kJ/mol��ϡ�����ϡ����������Һ��Ӧ���Ȼ�ѧ����ʽΪ��$\frac{1}{2}$H2SO4��aq��+NaOH��aq���T$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3 kJ•mol-1��
�ʴ�Ϊ��$\frac{1}{2}$H2SO4��aq��+NaOH��aq���T$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3 kJ•mol-1��
��2����4���¶Ȳ�ֱ�Ϊ��4.0�棬4.0�棬3.9�棬4.1�棬4�����ݶ���Ч���¶Ȳ�ƽ��ֵΪ4.0�棻
�ʴ�Ϊ��4.0��
��50mL 0.50mol•L-1 NaOH��Һ��30mL 0.50mol•L-1������Һ�����кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.50mol/L=0.025mol����Һ������Ϊ80ml��1g•cm-3=80g���¶ȱ仯��ֵΪ��T=4.0�棬������0.025molˮ�ų�������ΪQ=m•c•��T=80g��4.18J/��g•�棩��4.0��=13376J����1.3376kJ������ʵ���õ��к��ȡ�H=-$\frac{1.3376kJ}{0.025mol}$=-53.5kJ/mol��
�ʴ�Ϊ��-53.5kJ/mol��
��a��װ�ñ��¡�����Ч�����õ�����ƫС���к��ȵ���ֵƫС����a��ȷ��
b������NaOH��Һ����ʱ���ӿ̶ȣ���Һ�����ƫ��Ũ��ƫС����Ӧ���ɵ�ˮƫС���ų�������ƫС���к��ȵ���ֵƫС����b��ȷ��
c����ΰ�NaOH��Һ����ʢ�������С�ձ��У�����ɢʧ����õ�����ƫС���к��ȵ���ֵƫС����c��ȷ��
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ��������ʼ�¶�ƫ�ߣ���õ�����ƫС���к��ȵ���ֵƫС����d��ȷ��
�ʴ�Ϊ��a��b��c��d��
���� ���⿼���Ȼ�ѧ����ʽ�Լ���Ӧ�ȵļ��㣬��Ŀ�Ѷȴ�ע�������к��ȵĸ�������Ȼ�ѧ����ʽ����д�������Լ��ⶨ��Ӧ�ȵ��������⣮

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�| A�� | ���ӵĻ�ԭ��ǿ����Fe2+��Br-��Cl- | |
| B�� | ��a��2bʱ�����������ӷ�Ӧ��2Fe2++Cl2�T2Fe3++2Cl- | |
| C�� | ��a=bʱ����Ӧ�������Ũ�ȣ�c��Fe3+����c��Br-����c��Cl-��=1��2��2 | |
| D�� | ��3a��2bʱ�����������ӷ�Ӧ��2Fe2++4Br-+3Cl2�T2Fe3++2Br2+6Cl- |
| A�� | ����ʯ��ˮ�м���һ������ʯ�ң��¶��������ߣ�������Һ��pH���� | |
| B�� | ��ӦMgCl2��l��=Mg��l��+Cl2��g���ġ�H��0��S��0 | |
| C�� | ��Ũ�ȵ�ϵ��ϡ��Һ�����������Ƣ������Ƣ۴����̼�����ƣ����ǵ�PH��С�������е�Ϊ�ۢ٢ܢ� | |
| D�� | 0.1mol•L-1 Na2SO3��Һ�У�c��OH-��-c��H+���T2 c��H2SO3��+c��HSO3-�� |
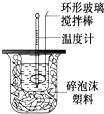 ������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�
������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�������Ͳ��ȡ50mL 0.25mol/L���ᵹ��С�ձ��У���������¶ȣ�
������һ��Ͳ��ȡ50mL 0.55mol/L NaOH��Һ��������һ�¶ȼƲ�����¶ȣ�
�۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ�������Һ����¶ȣ��ش��������⣺
��1��д��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ���к�����ֵΪ57.3kJ/mol����NaOH��aq��+$\frac{1}{2}$H2SO4��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
��2������NaOH��Һ����ȷ�����ǣ�C�� ��������ѡ����ѡ������
A���ز������������롡B����������������C��һ��Ѹ�ٵ���
��3��ʵ���������±���������д�±��еĿհף�
| �¶� ʵ����� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 29.5 | |
| 2 | 27.0 | 27.4 | 27.2 | 32.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.2 | |
| 4 | 26.4 | 26.2 | 26.3 | 29.8 | |
������ʵ����ֵ�����57.3kJ/mol��ƫ�����ƫ���ԭ������ǣ�����ĸ��abc��a��ʵ��װ�ñ��¡�����Ч����b�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�
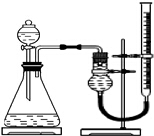 ̽��Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч��
̽��Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч��ʵ��С������ͼ��ʾװ�ã�ѡȡ����Լ�����Ʋ���������ʵ�飮�����������ص�Ӱ�죬ʵ����������ݼ�¼���±���
| ʵ����� | 5% H2O2��Һ�������mL�� | ѡ���Լ� | ѡ���Լ������mL�� | �ռ�V mL O2����ʱ�䣨min�� |
| �� | 10 | ����ˮ | 5 | m |
| �� | 10 | 0.1mol/L CuSO4 | v1 | n |
| �� | 10 | �Լ�M | 5 | p |
| �� | 10 | 0.1mol/L Na2SO4 | 5 | m |
��2��ʵ��٢ܵ���ҪĿ����֤����������Ӷ�H2O2�ֽ���Ч����
��3��ʵ�����ѡ���Լ������v1=5mL��
��4��ʵ�����ѡ�õ��Լ�M��Fe2��SO4��3������ȷѡ���Լ�����ʵ�飬����ռ�V mL O2����ʱ��m��n��p���ɴ˵ó���ʵ�������Fe3+��H2O2�ֽ�Ĵ�Ч����Cu2+��H2O2�ֽ�Ĵ�Ч�����ã�
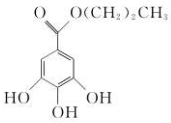 ûʳ��������Ľṹ��ʽΪ������ʪ�ԣ��Թⲻ�ȶ��������ֽ⣬�����Բ�����й�˵����ȷ���ǣ�������
ûʳ��������Ľṹ��ʽΪ������ʪ�ԣ��Թⲻ�ȶ��������ֽ⣬�����Բ�����й�˵����ȷ���ǣ�������| A�� | ����ʽΪC10H13O5 | B�� | ���Է���ȡ����Ӧ�ͼӳɷ�Ӧ | ||
| C�� | ������ˮ���Ҵ��������ܼ� | D�� | װ����ɫ����ƿ�У��ܷⱣ�� |