��Ŀ����
����Ŀ��(1)ijУ�о���ѧϰС���ѧ������ȡ���ռ���������̽���������й����ʣ��������ش�ʵ���е��й����⡣
��Ҫ�ռ��ϴ��ĸ��ﰱ����ʹ�õķ�����__________________________��
�ڼס�����С���ѧ������ͬ�ݻ���Բ����ƿ���ռ�һƿ���ﰱ��,����ʵ��.�������������Ȫ,˵������______����ˮ��Բ����ƿ����Һ���ɫ,˵����_____���������ɡ�
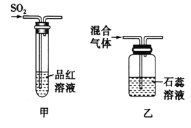
(2)ij��ȤС��Ϊ��֤SO2��Cl2��Ư����,��������·���,�������ش���������(β������װ��δ����)_____��
����ͼ����ʾ����Ʒ����Һ��ͨ��SO2,ͬѧ�Ƿ���Ʒ����Һ��ɫ��,ֹͣͨ����,�����Թ�,������Һ�ֱ�Ϊ��ɫ��˵��SO2��Ư����__________(�����ɻָ������������ɻָ�����)��
����ͼ����ʾ,�������Cl2��SO2���������1:1���,ͨ��ʯ����Һ��,����ʯ����Һ���,����ɫ,���û�ѧ����ʽ����֮:_____________________________________________________
���𰸡������ſ����� (��)�� �� �� �ɻָ��� SO2+Cl2+2H2O==H2SO4+2HCl
��������
��1���ٸ��ݰ����������ж����ռ�������
�ڼ������ڸ���Һ���������γ���Ȫ�����ݰ��������ʷ����жϣ�
��2���ٸ��ݶ��������Ư���ص�����жϣ�
�ڸ���������������������������
��1���ٰ�����������ˮ���ܶ�С�ڿ�������Ҫ�ռ��ϴ��ĸ��ﰱ����ʹ�õķ����������ſ�������
�ڰ�����������ˮ������ܲ�����Ȫ����������ˮ����һˮ�ϰ�����Һ�Լ��ԣ�����Բ����ƿ����Һ���ɫ��
��2���ٶ����������Ư������ʹƷ����Һ��ɫ�������������Ư���Dz��ȶ��ģ����Ⱥ���Һ�ָֻ���ԭ������ɫ�����˵��SO2��Ư���ǿɻָ��ġ�
����������ǿ�����ԣ��ܰѶ�����������Ϊ���ᣬͬʱ�����Ȼ��⣬�Ӷ�ʧȥƯ���ԣ����Խ������Cl2��SO2���������1:1��ϣ�ͨ��ʯ����Һ�У�����ʯ����Һ��죬������ɫ����Ӧ�ķ���ʽΪSO2+Cl2+2H2O��H2SO4+2HCl��

 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�