��Ŀ����
��������������Ⱦ��Ϊ���أ����������������ü�����⡣
(1)���������һ�������ȼҵ��Ʒ������������������ķ������������£�
(��)����SO2�ķ���ͨ���ⱥ��ʳ��ˮ�����õ�����Һ�У���NaHSO3��Һ��
(��)����ⱥ��ʳ��ˮ�������巴Ӧ���Ƶ����ᡣ
(��)���������NaHSO3��Һ�У���Ӧ���õ���SO2������գ����ɵ�NaClѭ�����á�
��д������(��)��Ӧ�Ļ�ѧ����ʽ�� ��
��д������(��)�е�ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ�� ��
��д������(��)��Ӧ�����ӷ���ʽ�� ��
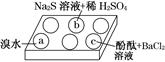
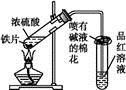
(2)����ѧ���������Fe2����Fe3�������ӵĴ����ã������½�SO2������SO42-��ʵ��SO2�Ļ������á�ij�о���ѧϰС��ݴ���������·�������ʵ���������²ⶨת������SO2������SO42-��ת���ʡ�
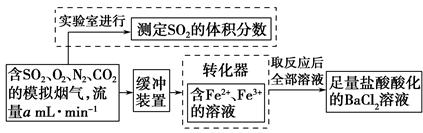
�ٸ�С�������ͼװ����ʵ���Ҳⶨģ��������SO2�����������X��Һ������ ��(��д���)
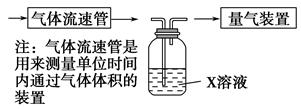
��������ʵ�����ڱ�״���½��еģ����ⶨת������SO2������SO ��ת���ʣ���֪�������٣�����ⶨ�������� �� ��
��ת���ʣ���֪�������٣�����ⶨ�������� �� ��
(1)���������һ�������ȼҵ��Ʒ������������������ķ������������£�
(��)����SO2�ķ���ͨ���ⱥ��ʳ��ˮ�����õ�����Һ�У���NaHSO3��Һ��
(��)����ⱥ��ʳ��ˮ�������巴Ӧ���Ƶ����ᡣ
(��)���������NaHSO3��Һ�У���Ӧ���õ���SO2������գ����ɵ�NaClѭ�����á�
��д������(��)��Ӧ�Ļ�ѧ����ʽ�� ��
��д������(��)�е�ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ�� ��
��д������(��)��Ӧ�����ӷ���ʽ�� ��
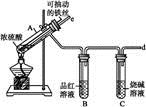
(2)����ѧ���������Fe2����Fe3�������ӵĴ����ã������½�SO2������SO42-��ʵ��SO2�Ļ������á�ij�о���ѧϰС��ݴ���������·�������ʵ���������²ⶨת������SO2������SO42-��ת���ʡ�

�ٸ�С�������ͼװ����ʵ���Ҳⶨģ��������SO2�����������X��Һ������ ��(��д���)

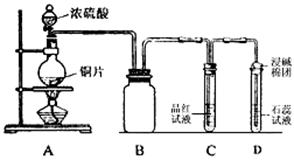
| A����ĵ�����Һ | B�����Ը��������Һ |
| C������������Һ | D���Ȼ�����Һ |
 ��ת���ʣ���֪�������٣�����ⶨ�������� �� ��
��ת���ʣ���֪�������٣�����ⶨ�������� �� ��(1)��SO2��NaOH=NaHSO3
��2NaCl��2H2O 2NaOH��H2����Cl2��
2NaOH��H2����Cl2��
��HSO3-��H��=SO2����H2O
(2)��A��B����ʵ��ʱ�䡡���������ữ��BaCl2��Һ�����ɳ���������
��2NaCl��2H2O
 2NaOH��H2����Cl2��
2NaOH��H2����Cl2����HSO3-��H��=SO2����H2O
(2)��A��B����ʵ��ʱ�䡡���������ữ��BaCl2��Һ�����ɳ���������
���SO2�ڻ�����е������������Ҫ�����������һ����SO2�������һ���ǻ���������������������װ�ã����������SO2�����������������ϴ��ƿ�е�X��Һ�����SO2����������Զ���ϴ��ƿ�е���Һ����������SO2��Ӧ�����ܷ������Ե���ɫ�仯���Ա�ȷ����Ӧ���յ㡣���SO2��ת���ʣ�Ӧ���SO2���������������SO��������Ϊ��ǰһ���������SO2�ڻ�����еĺ���������ֻ��ȷ�������������������������������ֻ��֪��ͨ��ʱ�䡣Ҫ��SO2��ת���ʣ�ֻ��ȷ������������

��ϰ��ϵ�д�
 �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
�����Ŀ

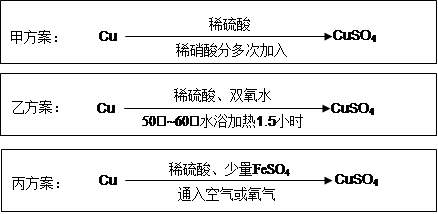
 CO2(g) ?H����283��0 kJ/mol
CO2(g) ?H����283��0 kJ/mol