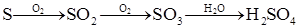��Ŀ����
ij��ѧС�����Na2SO3������ʵ��̽����
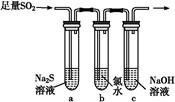
�ڰ�ɫ��ΰ��a��b��c���������е���Na2SO3��Һ���ٷֱ�μ���ͼ��ʾ���Լ���
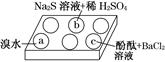
ʵ���������±���
����ʵ��������з�����
(1)a��ʵ������֤��Na2SO3����________�ԡ�
(2)b�з�����Ӧ�����ӷ���ʽ��_____________________________________________
(3)Ӧ�û�ѧƽ��ԭ������c������(�û�ѧ���P�����ֱ���)__________________________________________________________��
�ڰ�ɫ��ΰ��a��b��c���������е���Na2SO3��Һ���ٷֱ�μ���ͼ��ʾ���Լ���

ʵ���������±���
| ��� | ʵ������ |
| a | ��ˮ��ɫ |
| b | ��������ɫ���� |
| c | �����̪��Һ��죬�ټ���BaCl2��Һ����������Һ�ɫ��ȥ |
����ʵ��������з�����
(1)a��ʵ������֤��Na2SO3����________�ԡ�
(2)b�з�����Ӧ�����ӷ���ʽ��_____________________________________________
(3)Ӧ�û�ѧƽ��ԭ������c������(�û�ѧ���P�����ֱ���)__________________________________________________________��
(1)��ԭ�ԡ�(2)SO32-��2S2����6H��=3S����3H2O
(3)��Na2SO3��Һ�У�SO32-ˮ���Լ��ԣ�SO32-��H2O HSO3-��OH�������Ե����̪����Һ��죻�ڸ���Һ�м��� BaCl2��Ba2����SO32-=BaSO3��(��ɫ)������c(SO32-)��С��SO32-ˮ��ƽ�����ƣ�c(OH��)��С����ɫ��ȥ��
HSO3-��OH�������Ե����̪����Һ��죻�ڸ���Һ�м��� BaCl2��Ba2����SO32-=BaSO3��(��ɫ)������c(SO32-)��С��SO32-ˮ��ƽ�����ƣ�c(OH��)��С����ɫ��ȥ��
(3)��Na2SO3��Һ�У�SO32-ˮ���Լ��ԣ�SO32-��H2O
 HSO3-��OH�������Ե����̪����Һ��죻�ڸ���Һ�м��� BaCl2��Ba2����SO32-=BaSO3��(��ɫ)������c(SO32-)��С��SO32-ˮ��ƽ�����ƣ�c(OH��)��С����ɫ��ȥ��
HSO3-��OH�������Ե����̪����Һ��죻�ڸ���Һ�м��� BaCl2��Ba2����SO32-=BaSO3��(��ɫ)������c(SO32-)��С��SO32-ˮ��ƽ�����ƣ�c(OH��)��С����ɫ��ȥ��(1)a�з�����Ӧ�Ļ�ѧ����ʽΪ��Na2SO3��Br2��H2O=Na2SO4��2HBr��֤��Na2SO3���л�ԭ�ԡ�
(2)b�в�������ɫ������������Ӧ�����ӷ���ʽ��SO32-��2S2����6H��=3S����3H2O��
(3)�����̪��Һ�������ΪSO32-������ˮ�ⷴӦ��ʹ��Һ�Լ��ԣ�SO32-��H2O??HSO3-��OH�����ټ���BaCl2��Һ��Ba2����SO32-��Ӧ����BaSO3��������Ӧ������SO32-ʹ��Ũ�ȼ�С��ˮ��ƽ�������ƶ���OH��Ũ�ȼ�С����ɫ��ȥ��
(2)b�в�������ɫ������������Ӧ�����ӷ���ʽ��SO32-��2S2����6H��=3S����3H2O��
(3)�����̪��Һ�������ΪSO32-������ˮ�ⷴӦ��ʹ��Һ�Լ��ԣ�SO32-��H2O??HSO3-��OH�����ټ���BaCl2��Һ��Ba2����SO32-��Ӧ����BaSO3��������Ӧ������SO32-ʹ��Ũ�ȼ�С��ˮ��ƽ�������ƶ���OH��Ũ�ȼ�С����ɫ��ȥ��

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
�����Ŀ


 ��ת���ʣ���֪�������٣�����ⶨ�������� �� ��
��ת���ʣ���֪�������٣�����ⶨ�������� �� �� 8SO2+2Fe2O3,�÷�Ӧ�б�������Ԫ������������(��Ԫ�ط���)�����÷�Ӧת��2.75 mol����ʱ,���ɵĶ��������ڱ�״���µ����Ϊ��������L��
8SO2+2Fe2O3,�÷�Ӧ�б�������Ԫ������������(��Ԫ�ط���)�����÷�Ӧת��2.75 mol����ʱ,���ɵĶ��������ڱ�״���µ����Ϊ��������L�� 
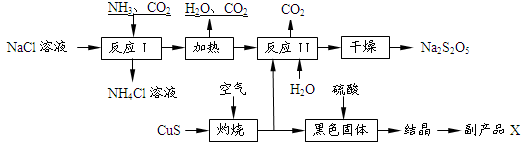
 Na2S2O5��H2O�ȶಽ��Ӧ��
Na2S2O5��H2O�ȶಽ��Ӧ��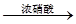 H2SO4
H2SO4