��Ŀ����
����Ŀ����1��ij��ԭ�ӵ�������a g����NAֻ��ʾ����٤����������ֵ����ԭ�ӵ����ԭ������Ϊ______��b g��ԭ�ӵ����ʵ���Ϊ______mol��
��2��a g A������b g B�����������ȣ�ͬ��ͬѹ�£�����A������B���ܶ�֮��Ϊ___________��
��3����10 mL 0.1mol/LNaHCO3��Һ�л�������0.1mol/LCa(OH)2��Һ�����ȷ�����Ӧ����������Ϊ____________�������ӷ��ţ���ͬ���������Һ���а�ɫ�������ɣ����ɳ�������������Ϊ____________������������Ca(OH)2��Һʱ�����������ӷ�Ӧ����ʽΪ_______________________��
��4�� ��3.22 gâ����Na2SO4��10H2O������ˮ�У�Ҫʹÿ100��ˮ����������1��Na+���������ˮ������Ϊ___g��
���𰸡�aNA ![]() a:b HCO3-��OH�� Ca2�� �� CO32- 2 OH��+ Ca2��+2 HCO3-=CaCO3��+ CO32-+2H2O 34.2
a:b HCO3-��OH�� Ca2�� �� CO32- 2 OH��+ Ca2��+2 HCO3-=CaCO3��+ CO32-+2H2O 34.2
��������
(1)��ijԭ�ӵ�������ag����NAֻ��ʾ�����ӵ���������ֵ�� ��1molԭ�ӵ�����Ϊ�� aNAg����Ħ������ΪaNAg/mol��b g��ԭ�ӵ����ʵ���Ϊ��![]() ��
��
�ʴ�Ϊ��aNA��![]() ��
��
(2)���ڱ�״���£�agA������bgB�����������ȣ� ����![]() ��֪��A������B�����Ħ������֮��Ϊa:b��ͬ��ͬѹ�������ܶ�֮�ȵ���Ħ������֮��Ϊ��a:b��
��֪��A������B�����Ħ������֮��Ϊa:b��ͬ��ͬѹ�������ܶ�֮�ȵ���Ħ������֮��Ϊ��a:b��
�ʴ�Ϊa:b ��
(3)����10 mL 0.1mol/LNaHCO3��Һ�л�������0.1mol/LCa(OH)2��Һ����ʱCa(OH)2��Һ�������������ȷ�����Ӧ����������Ϊ��HCO3-��OH����HCO3-��OH����Ӧ����CO32-���������ɳ�������������Ϊ��Ca2�� ��CO32-������������Ca(OH)2��Һʱ�����������ӷ�Ӧ����ʽΪ��2OH��+ Ca2��+2 HCO3-=CaCO3��+ CO32-+2H2O��
�ʴ�Ϊ��HCO3-��OH����Ca2�� ��CO32-��2OH��+ Ca2��+2 HCO3-=CaCO3��+ CO32-+2H2O��
(4)��3.22gâ�������ʵ���Ϊ![]() ��������Һ��n(Na+)= 2n(Na2SO4��10H2O)=0.01mol��2=0.02mol��ÿ100��ˮ����������1�������ӣ�����n(H2O)= 100n(Na+) = 2mol���к���ˮ��0.01mol Na2SO4��10H2O���ʵ���Ϊ0.01mol��10=0.1mol��������Ҫ��ˮ�����ʵ���Ϊ2mol-0.1mol=1.9mol��������Ҫˮ������Ϊ1.9mol x��18g/ mol= 34.2g��
��������Һ��n(Na+)= 2n(Na2SO4��10H2O)=0.01mol��2=0.02mol��ÿ100��ˮ����������1�������ӣ�����n(H2O)= 100n(Na+) = 2mol���к���ˮ��0.01mol Na2SO4��10H2O���ʵ���Ϊ0.01mol��10=0.1mol��������Ҫ��ˮ�����ʵ���Ϊ2mol-0.1mol=1.9mol��������Ҫˮ������Ϊ1.9mol x��18g/ mol= 34.2g��
�ʴ�Ϊ��34.2��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ�������̼����(Na2CS3)��ũҵ������ɱ������ɱ�߳�����ڹ�ҵ�����ڴ�����ˮ�е��ؽ������ӣ�ij��ѧ��ȤС���Na2CS3��һЩ���ʽ�����̽����ʵ�顣
ʵ���̽��Na2CS3�����ʣ�
���� | ���������� |
�� | ȡ����Na2CS3������������ˮ�У����Ƴ���Һ���ֳ����ȷ� |
�� | ������һ����Һ�еμӼ��η�̪�Լ�����Һ��ɺ�ɫ |
�� | ����һ����Һ�еμ��������ữ��KMnO4��Һ����ɫ��ȥ |
��1��H2CS3��___������ǿ�������������ᡣ
��2����֪������з�Ӧ������������SO42-����÷�Ӧ�����ӷ���ʽΪ��____��
��3��ijͬѧȡ����۷�Ӧ��������Һ���Թ��У��μ�����������Ȼ�����Һ������Ϊͨ���ⶨ�����İ�ɫ�����������������ʵ�������õ�Na2CS3�����������Ƿ�ͬ�����Ĺ۵㣬��˵�����ɣ�___��
ʵ��ⶨNa2CS3��Һ��Ũ�ȣ�
����ͼ��ʾװ�ý���ʵ�飺��50.0mLNa2CS3��Һ����������ƿ�У�������M�Ļ�������������2.0mol��L-1��ϡH2SO4���رջ�����
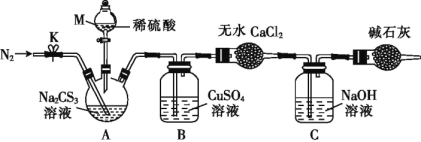
��֪��CS32- +2H+��CS2+H2S����CS2��H2S���ж�����CS2������ˮ���е�Ϊ46�棬��CO2��ijЩ�������ƣ���NaOH��������Na2COS2��H2O��
��4������M��������___����Ӧ��ʼǰ��Ҫ��ͨ��һ��ʱ��N2��������Ϊ____��
��5��B�з�����Ӧ�����ӷ���ʽΪ��____��
��6��Ϊ�˼��������̼������Һ��Ũ�ȣ���ͨ���ⶨB�����ɳ��������������㡣����B�г�������֮ǰ��Ҫ���е�ʵ�������___����B�����ɳ���������Ϊ8.4g����Na2CS3��Һ�����ʵ���Ũ����____��