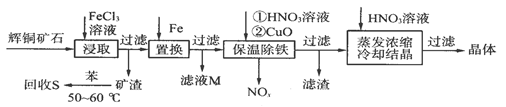
��Ŀ����
(14��)
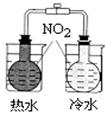
ij�о�С��Ϊ̽���������������������绯ѧ��ʴ���͵�Ӱ�����أ�����Ͼ��ȵ��������ۺ�̼��������ƿ�ײ�������ƿ��(��ͼ1)���ӽ�ͷ�ι��е��뼸�δ�����Һ��ͬʱ���������е�ѹǿ�仯��
��1�����������ʵ����Ʊ�(���в�Ҫ���ո�)��
| ��� | ʵ��Ŀ�� | ̼��/g | ����/g | ����/% |
| �� | Ϊ����ʵ�������� | 0.5 | 2.0 | 90.0 |
| �� | ����Ũ�ȵ�Ӱ�� | 0.5 | | 36.0 |
| �� | | 0.2 | 2.0 | 90.0 |
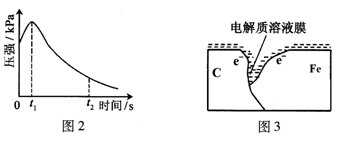
��3����С���ͼ2��0��t1ʱѹǿ����ԭ����������¼��裬������ɼ������
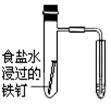
����һ���������ⸯʴ���������壻
������� ��
����
��4��Ϊ��֤����һ��ijͬѧ����˼����ռ����������Ƿ���H2�ķ��������������һ��ʵ�鷽����֤����һ��д��ʵ�鲽��ͽ��ۡ�
| ʵ�鲽��ͽ���(��Ҫ��д�����������)�� |
��1����2.0 ��̼�ۺ�����Ӱ��
��2��������ʴ  ��ԭ��Ӧ 2H2O+O2+4e-=4OH- ����4H++O2+4e-=2H2O��
��ԭ��Ӧ 2H2O+O2+4e-=4OH- ����4H++O2+4e-=2H2O��
��3����Ӧ���ȣ��¶����ߣ��������
��4��ʵ�鲽��ͽ��ۣ���Ҫ��д����������̣�
��ҩƷ�����Ͳ���ͬ��Ţ�ʵ�飨�����Ƥ�����ӽ��������ܣ�
��ͨ������ž�ƿ�ڿ�����
�۵��������Һ��ͬʱ����ƿ��ѹǿ�仯��Ҳ�ɲ��¶ȱ仯������Fe2+�ȣ���
���ƿ��ѹǿ������һ�������������һ��������
���������ڿ��������⣬�����𰸾����֣�
���������������1��̽��Ӱ�컯ѧ��Ӧ���ʣ�ÿ��ֻ�ܸı�һ�����������Т������������䣬Ϊ2.0g�����иı���̼�۵���������Ϊ̽��̼�۵��������ʵ�Ӱ�졣
��2��ѹǿ����������ʵ��������ȣ���ͼ�п��Կ��������������ʼ���ӣ�����٣���Ϊ������ʴ�����ý�������������̼Ϊ������������ԭ��Ӧ��
��3���������Ӱ���������֣��¶����ߣ��������
��4�����ڼ���һ����֪������������������Щ�仯���ӱ仯���ֿ���
���㣺ʵ�������̽����Ӱ�컯ѧ��Ӧ���ʵ����ء�

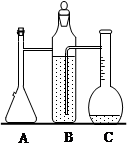
��ͼ��ʾ��ʵ�飬�ܴﵽʵ��Ŀ�ĵ���
| A | B | C | D |
 |  |  | 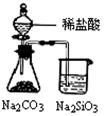 |
| ��֤��ѧ��ת��Ϊ���� | ��֤�¶ȶ�ƽ���ƶ���Ӱ�� | ��֤���������ⸯʴ | ��֤�ǽ�����Cl�� C �� Si |
����ͼʾʵ���������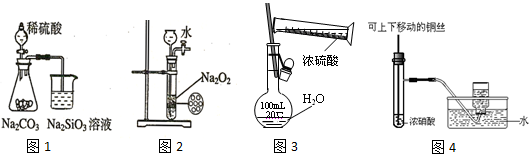
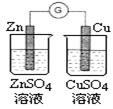
| A��ͼ1Ϊ֤���ǽ�����ǿ����S>C>Si | B��ͼ2Ϊ�Ʊ��������� |
| C��ͼ3Ϊ����һ��Ũ��������Һ | D��ͼ4�Ʊ����ռ�����NO2���� |
����ʵ���ܴﵽĿ�ĵ���
| A����п��ϡ�����Ʊ�H2 |
| B��������KMnO4��Һ����1-��ϩ�ͼױ� |
| C���ö����ЧӦ��������Һ��ʳ��ˮ |
| D����ˮ����ȥNO2��������NO |
���������Ҫ�ɷֿɱ�ʾΪFeO��Cr2O3��������MgO��Al2O3��Fe2O3�����ʣ��������Ը�����Ϊԭ���Ʊ��ظ���أ�K2Cr2O7��������ͼ��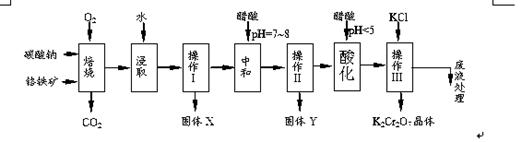
��֪����4FeO��Cr2O3+ 8Na2CO3+ 7O2 8Na2CrO4 + 2 Fe2O3 + 8CO2����
8Na2CrO4 + 2 Fe2O3 + 8CO2����
��Na2CO3 + Al2O3 2NaAlO2 + CO2������Cr2O72��+ H2O
2NaAlO2 + CO2������Cr2O72��+ H2O
 2CrO42�� + 2H+
2CrO42�� + 2H+
��������ش��������⣺
��1������X����Ҫ����_________����д��ѧʽ����Ҫ����ữ��������Һ��pH�Ƿ����4.5��Ӧ��ʹ��__________����д�������Լ����ƣ���
��2���ữ�����ô��������ҺpH<5����Ŀ����_________________________________��
��3���������жಽ��ɣ����K2Cr2O7����IJ��������ǣ�����KCl���塢����Ũ���� �����ˡ�_______�����
��4���±���������ʵ��ܽ�����ݣ�����������Ӧ�Ļ�ѧ����ʽ�ǣ�Na2Cr2O7+2KCl ��K2Cr2O7��+2NaCl��
| ���� | �ܽ��/(g/100gˮ) | ||
| 0��C | 40��C | 80��C | |
| KCl | 28 | 40.1 | 51.3 |
| NaCl | 35.7 | 36.4 | 38 |
| K2Cr2O7 | 4.7 | 26.3 | 73 |
| Na2Cr2O7 | 163 | 215 | 376 |
�÷�Ӧ����Һ���ܷ�����������_______________��
��5������ƷY��Ҫ��������������������þ���������ܻ����P���������ʣ���ȷ����Y���������������ķ����dz�ȡn g��Ʒ���������______����д�Լ������ܽ⡢���ˡ���______����д�Լ��������ա���ȴ���������ø������m g ��������Ʒ��������������������Ϊ_________���ú�m��n�Ĵ���ʽ��ʾ����
��1��ijѧϰС��������ͼװ����ȡ������̽�������ʡ�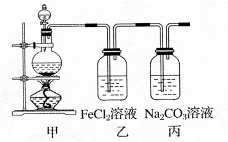
�ټ�װ���з�Ӧ�Ļ�ѧ����ʽ�� ��
��֤����װ����FeCl2��Һ��Cl2�����˷�Ӧ��ʵ�鷽���ǣ�ֻע���Լ������� ��
�۱�װ����ͨ������Cl2�����Ƶ�ij�������г��õ�Ư�ס����������ʡ���֪̼�������ǿ�ڴ����ᣬ����з�Ӧ�Ļ�ѧ����ʽ�� ��
��2����һƿ���ڷ��õ�Ư�ۣ������������������Լ�����ɸ�Ư�۳ɷݵ�̽����
�Թܡ���ͷ�ιܡ������ܵĵ�����������ˮ��1mol��L-1���ᡢƷ����Һ�����Ƴ���ʯ��ˮ��
��������衿����һ����Ư��δ���ʣ���CaCl2��Ca��ClO��2��
���������Ư��ȫ�����ʣ��� ��
����������Ư�۲��ֱ��ʣ���CaCl2��Ca��ClO��2��CaCO3 ��
������ʵ�顿�ڴ��������±������ؼ���Ca2+��Cl-����
| ʵ�鲽�� | Ԥ������ͽ��� | |
| �� | ��A�Թ�ȡ��������ʯ��ˮ���ã���B�Թ�ȡ������Ʒ������B�Թ� | ��������ų��ҳ���ʯ��ˮδ�����ǣ������һ������ |
| �� | | |
��14�֣�
ij��ѧ��ȤС��Ϊ̽��Cl2��Br2��Fe3+��������ǿ�������������ʵ�飺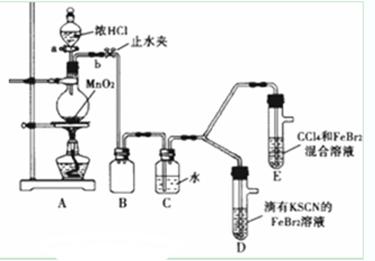
��1�� �ټ�����巢��װ��A�������ԵIJ����ǣ��ߣߣߣߣߣߣߣߣߣߣߣ�
������ʵ��װ�ô���һ�����Բ��㣬��ָ���ߣߣߣߣߣߣߣߣߣߣߣߣߣ�
��2�� �ø������װ�ý���ʵ�飮ʵ��������£�
| ʵ����� | ʵ������ | ���� |
| ����a����Բ����ƿ�е�������Ũ���Ȼ��رջ���a����ȼ�ƾ��� | Dװ���У���Һ��� Eװ���У�ˮ����Һ�����CCl4�������Ա仯 | Cl2��Br2��Fe3+����������ǿ������˳��Ϊ�� ______________________ |
Dװ���У���ɫ������ȥ��Eװ���У�CCl4��������ɫ��Ϊ��ɫ������ɫ��������ɺ�ɫ��Ϊ̽������ʵ������ı��ʣ�С��ͬѧ����������£�
| ��SCN��2������±�ص������ƣ������ԣ�Cl2����SCN��2�� ��Cl2��Br2��Ӧ����BrCl�����ʺ�ɫ���Դ���ɫ�����е�Ϊ5�棬��ˮ����ˮ�ⷴӦ�� ��AgClO��AgBrO��������ˮ�� |
�� ��̽��E����ɫ�仯��ԭ�����ʵ�����£��÷�Һ©�������E���²���Һ�������ռ���ɫ���ʣ�ȡ����������AgNO3��Һ������۲쵽���а�ɫ�������������ϻ�ѧ������ͽ�������ɫ������ԭ��ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
�۽�����BrClͨ�뵽�⻯����Һ�У��÷�Ӧ�Ļ�ѧ����ʽΪ���ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�