��Ŀ����
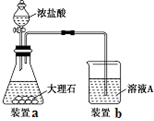
ijͬѧ����������ⶨ������þ�����ᷴӦ�ⶨ1 mol������������������գ�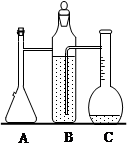
��1��A�з�����Ӧ�����ӷ���ʽΪ______________________��
��2�����װ�������Եķ���������Bƿ�IJ�����������Ƥ������Aƿ���Ͽڣ�������__________________����ʱ������ȷ��װ�����������á�
��3����֪Һ����ƿ�Ŀ̶ȷ�Χ��110~130 mL��ʵ��ʱ��ȡþ��������Ҫ������0.100~0.110 g֮�䣬Ŀ����_________��
��4�����һ�βⶨʵ�飬��Ҫ2����ע����������������Ҫ��¼���ǵ�__________�γ������������
��5����������ᵼ��ʵ����ƫ�ߵ���______�����ţ�
a. þ���������Ĥû�г��� b. ��Һƿ�е�Һ����ˮ
c. δ��ȴ�����¾Ͷ��� d. װ�������Բ���
��1��Mg+2H+��Mg2++ H2����2�֣��� ��2��B�е�����Һ������������һ�����ڲ��½�����3��ȷ��������������ڿ̶ȷ�Χ֮�ڡ� ��4��2�� ��5��c��
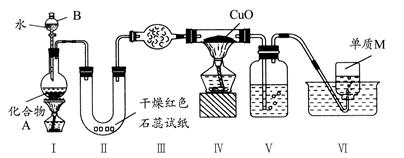
��������������������Ʒֱ�Ϊ��A���巢������B��Һƿ��CҺ����ƿ��
��1��A����þ�����ᷴӦ��������þ�����������ݲ���д�����ӷ���ʽ��
��2��װ�������Կ�������װ��������ѹǿ�仯��Һ��仯�����ж�װ�������ԣ�
��3��ȡ110��30 mL��������ֵ������������������ʵ��������������ʵ�����þ�����ʵ�����ȣ������þ���������ͻ�õ���Χ0.100~0.110 g����ô����Ŀ�ĵ�Ȼ��ȷ��������������ڿ̶ȷ�Χ֮�ڡ�
��4��ÿ����ʵ��ʱ����ע������Aƿ���Ͽڳ�����ʹBƿ������Һ���ƽ��Bƿ������ѹǿ��������ѹ��ȣ���ע��ע�����γ�ʱҪ����ͷ�γ�����ʱ����ⶨ��ʼ״̬���ٴγ�����ѹ����ע������Aƿ���Ͽڴ�������ʹBƿ��Һ���ƽ������ʼ״̬��ͬ������ע�����г��������������¼��ֵ������ÿ������Ҫ��2��ע����������ƽ����3��ʵ�鹲��ע��������6�Ρ�
��5��a. þ�������������û�г�����������������ᷴӦ��û���������ɣ��ᵼ���������ƫС���ʴ���
b. ��Һƿ�е�Һ����ˮ���ᵼ���������ƫС���ʴ���
c.δ��ȴ�����¾Ͷ��������������������������ƫ����ȷ��
d. װ�������Բ��ã���Ȼ�ᵼ���������ƫС���ʴ���
���㣺����ʵ����Ʋⶨ������������ķ����Ͳ��衣

(14��)
ij�о�С��Ϊ̽���������������������绯ѧ��ʴ���͵�Ӱ�����أ�����Ͼ��ȵ��������ۺ�̼��������ƿ�ײ�������ƿ��(��ͼ1)���ӽ�ͷ�ι��е��뼸�δ�����Һ��ͬʱ���������е�ѹǿ�仯��
��1�����������ʵ����Ʊ�(���в�Ҫ���ո�)��
| ��� | ʵ��Ŀ�� | ̼��/g | ����/g | ����/% |
| �� | Ϊ����ʵ�������� | 0.5 | 2.0 | 90.0 |
| �� | ����Ũ�ȵ�Ӱ�� | 0.5 | | 36.0 |
| �� | | 0.2 | 2.0 | 90.0 |
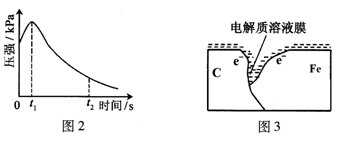
��3����С���ͼ2��0��t1ʱѹǿ����ԭ����������¼��裬������ɼ������
����һ���������ⸯʴ���������壻
������� ��
����
��4��Ϊ��֤����һ��ijͬѧ����˼����ռ����������Ƿ���H2�ķ��������������һ��ʵ�鷽����֤����һ��д��ʵ�鲽��ͽ��ۡ�
| ʵ�鲽��ͽ���(��Ҫ��д�����������)�� |
[ʵ�黯ѧ]
��������FePO4��2H2O��������ˮ���װ�ɫ���壩����������ҩ�ʳƷ���Ӽ�������ӵ�ص��������ϣ�ʵ���ҿ�ͨ������ʵ���Ʊ���������
��1����ȡһ�����ѳ�ȥ���۵ķ���м�������Թ�����ϡ���ᣬ���ȡ����裬��Ӧһ��ʱ�����ˣ���Ӧ���ȵ�Ŀ���� ��
��2������Һ�м���һ����H2O2����Fe2����Ϊȷ������H2O2������������K2Cr2O7����Һ�ζ���Һ�е�Fe2�������ӷ���ʽ���£�
������ζ���ע��K2Cr2O7����Һǰ���ζ�����Ҫ��©�� �� ��
�����ζ�xmL��Һ�е�Fe2��������amol��L��1 K2Cr2O7����ҺbmL������Һ��
c(Fe2��)= mol��L��1
��Ϊʹ��Һ�е�Fe2����ȫ��H2O2����������ʵ������������ȷ���� ������ţ���
| A�������ʵ�������H2O2��Һ | B�������μ�H2O2��Һ������ |
| C�����ȣ�ʹ��Ӧ�ڽϸ��¶��½��� | D���ð�ˮ����pH=7 |
����һƿʵ���ҷ����ѾõĿ��ܱ�������Na2SO3���壬Ϊ���о�������ɣ��������ͬѧ�ǽ��е�����̽�����
��ѡ���Լ���ŨH2SO4��ŨHNO3��10%���ᡢ0.1mol/LH2SO4��0.1mol/LHNO3��0.1mol/LBaCl2��0.1mol/LBa(NO3)2��3%H2O2��10%NaOH��Һ������ˮ��Ʒ����Һ��������ѡ��
��1���������
����һ������ȫ����Na2SO3�� �����������ȫ����Na2SO4��
�������� ��
��2�����ʵ�鷽��(��)��ѡ����ͼװ�ý���ʵ�飬��װ�õ��ŵ��� ��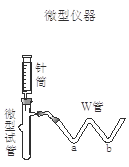
��3������ʵ�飺�����±����ü�Ҫ����д��ʵ�������Ԥ������ͽ��ۡ�
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����������Ʒ�����Թ��У���W��a������ ��b������ ���ý��ܽ�W�������Թ����Ӻ� | |
| ����2������Ͳ���� ������ͷ�������ԹܵĽ������������Ʒ��ע�����Һ�� | �� |
| ����3��������Ͳ����������ˮϴ���������� ע�����Թ��� | �� |
��4����������̽�����������ɵ�����ͨ�뵽����������Ư��Ũ��Һ�У�������ɰ�ɫ��������д���÷�Ӧ�����ӷ���ʽ�� ��
��ѧ������������������ɳ�����չ������ء�����˵����ȷ����
| A���߲˱����������������֣��ɱ��ʺ����� |
| B����ɫʳƷ���Dz�ʹ�û���ũҩ�������κλ�ѧ���ʵ�ʳƷ |
| C���ƹ�����TiO2�ⴥý�������衰��̬��·����������β��ת��Ϊ������ |
| D���ƹ�ʹ��úҺ�������ɼ��ٶ�����̼��������������ŷ� |