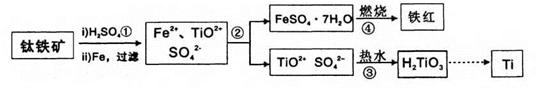
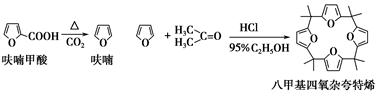
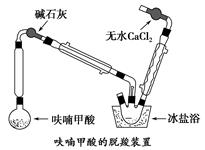
��Ŀ����
��ȸʯ��Ҫ��Cu2(OH)2CO3,��������Fe��Si�Ļ����ʵ�����Կ�ȸʯΪԭ���Ʊ�CuSO4��5H2O��CaCO3���������£�
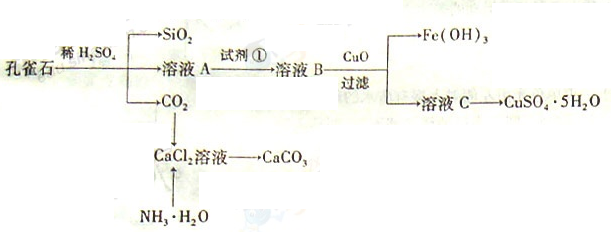
��ش��������⣺
��1����ҺA�Ľ���������Cu2+��Fe2+��Fe3+�������������Լ���ѡ��ʵ�鲽�����Լ���Ϊ______������ţ���������ҺA��Fe3+������Լ�Ϊ��������������ţ���
a��KMnO4����������b��(NH4) 2S c��H2O2 d��KSCN
��2������ҺC���CuSO4��5H2O����Ҫ���������������������� �����˵Ȳ��������ձ���©���⣬���˲������õ���һ�����������������ڴ˲����е���Ҫ�������������� ��
��3���Ʊ�CaCO3ʱ��Ӧ��CaCl2��Һ����ͨ�루���ȼ��룩�������� ���ѧʽ������ʵ��������а����ݳ���Ӧѡ�������������� װ�û��գ�����ţ���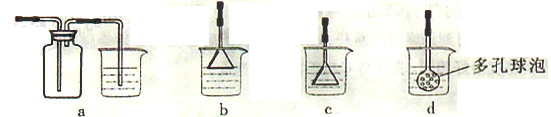
��4�����ⶨ��ҺA��Fe2+��Ũ�ȣ���Ҫ������ƿ����ij����Һ������ʱ����Ӧ ��ֱ�� ����KMnO4����Һ�ζ�ʱӦѡ�����������ζ��ܣ����ʽ����ʽ������
��1�� c (2��) d (2��) ��2����ȴ�ᾧ (2��) ���� (2��)
��3��NH3��H2O (2��) b (2��)
��4 ƽ�Ӱ�Һ�棨��ƽ�ӿ̶��ߣ���1�֣� ��Һ�����͵���̶������� (1��) ��ʽ (1��)
���������������1��Ҫ��ȡ����ͭ���壬�ͱ����ȥ��Һ�е��������ӣ����ó�������ȥ�����ӡ��������Ҫ����Һ�е����������������������ӣ���ͬʱ�ֲ��������µ����ʣ�˫��ˮ�Ļ�ԭ������ˮ�������������ʡ����Դ�ѡC��������ҺA��Fe3��������Լ���KSCN��Һ����2��Ҫ���CuSO4��5H2O����Ҫͨ��������������ȴ�ᾧ��Ȼ����˼��ɡ�����ʱ���ձ���©���⣬����Ҫ��������������3������CO2���ܽ�Ⱥ�С����������ͨ�백����ʱ��Һ�Լ��ԣ�Ȼ����ͨ��CO2���õ�̼��ƹ��塣���ڰ�����������ˮ������������Ҫ������ֹ��������˴�ѡb����4�����ⶨ��ҺA��Fe2+��Ũ�ȣ���Ҫ������ƿ����ij����Һ������ʱ����Ӧƽ�Ӱ�Һ�棨��ƽ�ӿ̶��ߣ���ֱ����Һ�����͵���̶������� ������KMnO4��Һ��ǿ�������ܸ�ʴ�ܣ��ζ�ʱӦѡ����ʽ�ζ��ܡ�
���㣺���⿼���������ӵļ��飬���ʵķ����ᴿ����ѡ������İ�ȫ���ռ��ζ�������

��������������Ԫ��֮һ���С�����Ԫ�ء�֮�ơ�ʳ�üӵ�ʳ�ο�Ԥ����ȱ��������������֪���������������£�I- �ܱ�NO3- ������IO3-����H2O2��O2������ΪI2���� IO3- �ܱ�HSO3- ��ԭ��I2��
��������ʵ���ҳ���������������ѡ�Լ��������о�ijʳ����Ʒ�����ӵ�Ĵ�����ʽ��I2��I-��IO3- �е���һ�֡�
��ѡ�Լ����£�1.0 mol?L-1HNO3��Һ��1.0 mol?L-1H2SO4��Һ��1.0 mol?L-1NaHSO3��Һ��3%H2O2��Һ��1%������Һ������ˮ��
��1���������
����1����ʳ����Ʒ�к�I2��
����2����ʳ����Ʒ�к�I-��
����3�� ��
��2����Ʒ�����ʵ��̽��
������ʳ����Ʒ��������ˮ�Ƴ���Һ���밴Ҫ����д�±���
| ʵ�鲽�� | Ԥ����������� |
| ����1��ȡ����������Һע���Թ��У����뼸�ε�����Һ���� | ����Һ�� ������ɫ���������1������������1���������ٽ��в���2 |
| ����2�� | ����Һ����ɫ�������2��������Ӧ�����ӷ���ʽΪ ��������2���������ٽ��в���3 |
| ����3�� | |
��3��������˼��
��KIO3��KI��������Ϊʳ�üӵ����е����Դ���ӻ�ѧ�Ƕ�������ʵ�������� ���KIO3����KI�������ã������� ��
����ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ������Ӻ�������ȡ����������£�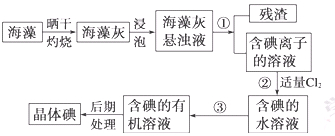
��1��ָ�������۵����ƣ� ���������г�������Cl2��Ŀ���� ��
��2����ȡ��Ĺ����У��ɹ�ѡ����Լ��� ( )
| A���ƾ� | B�����Ȼ�̼ | C������ | D������ |
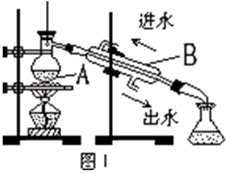
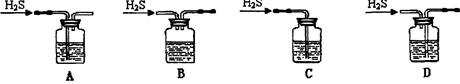
��3���Ӻ�����л���Һ����ȡ��ͻ����л��ܼ����辭������ͼI��ijͬѧ��Ƶ�����װ�ã�ͼ�����ԵĴ����� ��
��4����ͬѧ��Ϊ����ʱ���ʹ��ˮԡ���ȣ�ʹ��ˮԡ���ŵ��ǣ� ����������������ۼ��� �����������ƣ��
��5�� ʵ��۷�������ķ�Һ�к���Cl�C��SO42�C����ֻȡһ����Һ����μ����Cl�C��SO42�C�����μ����Լ���Ϊ�� �� ��
��6��ijС��ͬѧʵ��ʱ���õ�һ�����ʵ���Ũ�ȵĵ�ˮ��Һ225mL������ʱ��Ҫ����������ƽ�����������ձ��⣬���� �� ����ҡ��ʱ������Һ����ڿ̶��ߣ��������ҺŨ������ƫ��ƫС����Ӱ�죩��
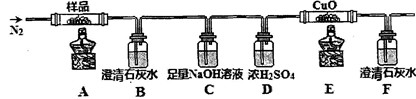
��1���������廯�ƺͽ�ŨH2SO4�����Ϊԭ�ϣ���ʵ�����Ʊ�1���嶡�飬�����鷴Ӧ�IJ��ָ��������֪��NaCl+H2SO4(Ũ)=NaHSO4+HCl�������������װ�ã����мг�������������������ȴˮ��û�л�������ش��������⣺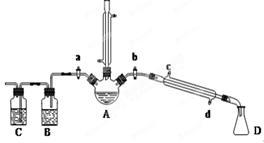
��1������D�������� ��
��2���ر�a��b����ͨ��ֱ�����ܵ�����ˮ����A����30���ӣ��Ʊ�1���嶡�顣д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3�������ϣ�������Ӧ�������ﻹ�����У����ѡ�1����ϩ���廯��ȡ�Ϩ��A���ƾ��ƣ�����ֱ�������Ϸ��������ӣ���a���������ȼ�����Ӧֱ����ȴ��ͨ��B��Cװ�ü��鲿�ָ����B��C��Ӧʢ�ŵ��Լ��ֱ��� �� ��
��4����ʵ������У�����A��Һ������ɫ��ɺ�ɫ���ú�ɫ������Ũ���ᷴӦ�Ļ�ѧ����ʽΪ ��������ֱ�����ܵ��϶�����һ����װ���ռ���ʯ�ҵĸ���ܣ�������Ⱦ������
��5������л�����������£�
| ���� | �۵�/0C | �е�/0C |
| 1������ | -89��5 | 117��3 |
| 1���嶡�� | -112��4 | 101��6 |
| ���� | -95��3 | 142��4 |
| 1����ϩ | -185��3 | -6��5 |
��6����ʵ������ȡ1��������NaBr�ֱ�Ϊ7��4 g��13��0 g�������Ĵֲ��ᆳϴ�ӡ�������ٴ�����õ�9��6 g 1���嶡�飬��1���嶡��IJ����� ��
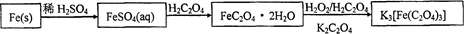
 ��Һ
��Һ
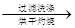 �ù���b g
�ù���b g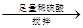
 ��������������Va mL
��������������Va mL 250 mL��Һ
250 mL��Һ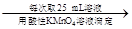 ����ƽ������0.1 mol��L��1����KMnO4��ҺVb mL����Ϊ���Ϸ����� ��ȷ����Ʒ����ɣ������� ��
����ƽ������0.1 mol��L��1����KMnO4��ҺVb mL����Ϊ���Ϸ����� ��ȷ����Ʒ����ɣ������� ��
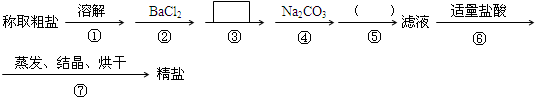
 ����д��ʹ�ó����Լ��Ļ�ѧʽ__________���ڣ� ���еIJ���������____ ___��
����д��ʹ�ó����Լ��Ļ�ѧʽ__________���ڣ� ���еIJ���������____ ___��