��Ŀ����
ij��ˮ�п��ܴ��ڵ��������£�Na+��Ag+��Ba2+��Al3+��AlO2һ��CO32����S2һ��SO32����SO42��
��ȡ����Һ�����й�ʵ�飬ʵ����̼��������£�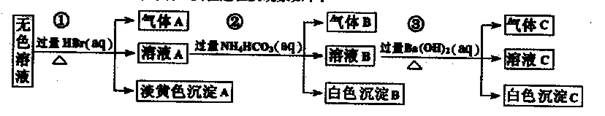
����˵����ȷ����
| A������ʵ����е�������Ƴ�������Aһ���Ǵ��������ɫ����һ����AgBr |
| B������ʵ����е�������Ƴ�������B��CO2������B��Al(OH)3��ԭ��Һ��һ������Al3+ |
| C������ʵ����е�������Ƴ�������C��NH3������Cһ����BaCO3��������BaSO4 |
| D��ԭ��Һ�п϶�����Na+��AlO2����S2��������ȷ���Ƿ���SO32����SO42�� |
C
����

��ϰ��ϵ�д�
�����Ŀ
���н�����ʵ�����ӷ���ʽ����ȷ����
A���Ȼ�ͭ��Һ�����ԣ�Cu2++2H2O Cu(OH)2+2H+ Cu(OH)2+2H+ |
| B������ʹʪ��ĵ���KI��ֽ������Cl2+2I-="2" Cl-+I2 |
| C������������Һ�м��백ˮ���ְ�ɫ��״������Al3++3OH-=Al(OH)3�� |
| D��ʵ���Ҳ��ò������Լ�ƿʢװ����������Һ��SiO2+2 OH-= SiO32-+H2O |
�������ӷ���ʽ��д��ȷ����
| A������Ͷ�뵽NaOH��Һ�У�2Al+2OH��= 2AlO2��+H2�� |
| B��AlCl3��Һ�м��������İ�ˮ��Al3++ 3OH�� = Al(OH)3�� |
C�����Ȼ�����Һ�м������ۣ� |
| D��FeCl2��Һ��Cl2��Ӧ��2Fe2++Cl2=2Fe3++2Cl�� |
����������ʵ�ķ���ʽ����ȷ����
| A�����������Һ��ͨ�������������Ca2+ + 2ClO��+ H2O + SO2��CaSO3��+ 2HClO |
| B���������������һ��ʱ����Һ��pH�½���2H2SO3��O2��2H2SO4 |
C������Һ������ϴ���۵�ԭ��CO32¯+H2O HCO3¯+OH¯ HCO3¯+OH¯ |
D����K2Cr2O7��Һ�м�������NaOHŨ��Һ����Һ�ɳ�ɫ��Ϊ��ɫ��Cr2O72��+H2O 2CrO42��+2H+ 2CrO42��+2H+ |
���������У����ڵ���ʵ���
| A������ | B������ͭ | C������������Һ | D���Ȼ��� |
��֪����ƽ�ⳣ����H2CO3��HClO��HCO�������ԣ�HClO��Cl2��Br2��Fe3����I2�������й����ӷ�Ӧ�����ӷ���ʽ�������У���ȷ����
| A����FeI2��Һ�еμ�������ˮ����Ӧ�����ӷ���ʽΪ2Fe2����Cl2=3Fe3����2Cl�� |
| B������ˮ�м��������Ȼ�������Һ��ʹ��ˮ�����ɫ |
C����NaClO��Һ��ͨ������CO2�����ӷ���ʽ��2ClO����CO2��H2O=2HClO�� |
| D����ʹpH��ֽ�����ɫ����Һ��Fe3����Cl����Ba2����Br���ܴ������� |
���з�Ӧ��Ӧ�����ӷ���ʽ��д��ȷ���ǣ� ����
| A��Ư��¶���ڿ�����ʧЧ��ClO����CO2��H2O=HClO��HCO3�� |
| B������������Һ������������Һ��Ӧ����Һǡ��Ϊ���ԣ�Ba2����H����SO42����OH��=BaSO4����H2O |
| C��Na2O2��H2O��Ӧ�Ʊ�O2��Na2O2��H2O=2Na����2OH����O2�� |
| D����ǿ������Һ�д���������Fe(OH)3��Ӧ����Na2FeO4��4OH����3ClO����2Fe(OH)3=2FeO42����3Cl����5H2O |
����������ӷ���ʽ��д��ȷ���� (����)��
| A��NaClO��Һ��FeCl2��Һ��ϣ�6Fe2����3ClO����3H2O=2Fe(OH)3����3Cl����4Fe3�� |
| B����ʳ���������е�̼��ƣ�CaCO3��2H��=Ca2����CO2����H2O |
| C��FeCl2������Һ���ڿ����б��ʣ�2Fe2����4H����O2=2Fe3����2H2O |
D�����MgCl2ˮ��Һ�����ӷ���ʽ��2Cl����2H2O H2����Cl2����2OH�� H2����Cl2����2OH�� |
������CO2ͨ��������Һ�У��������ӻ��ܴ���������ǣ� ��
| A��K����SiO32-��Cl����NO3- |
| B��H����NH4+��Al3����SO42- |
| C��Na����S2����OH����SO42- |
| D��Na����CO32-��CH3COO����HCO3- |