��Ŀ����
5��25��ʱ��������������Һ����0.1mol/L CH3COOH��Һ�� ��pH=13NaOH��Һ����0.05mol/L H2SO4�� ��0.1mol/L Na2CO3��Һ���� ��Mg��OH��2�ı�����Һ�����Ҫ����д���пհף�
��1����Һ�ܳʼ��ԣ�����ԡ��������ԡ������ԡ�������ԭ����CO32-+H2O=HCO3-+OH-�������ӷ���ʽ��ʾ��
��2��������������������Һ�У�pH�ɴ�С��˳��Ϊ�ڢܢ٢ۣ�����ţ�
��3�������¶���amL����Һ����bmL����Һ�ۻ�ϣ����û����Һ��pH=7����a��b=1��1��
��4�����������Һ�������ϣ�������Һ����Ũ���ɴ�С��˳��c��Na+����c��CH3COO-����c��HCO3-����c��OH-����c��H+����c��CO32-����
��5������Һ���м�������NaOH���壬����pH=13����þ����Ũ��Ϊ5.6��10-10mol/L������֪Mg��OH��2�ڸ��¶��µ��ܶȻ�����Ϊ5.6��10-12��
���� ��1��̼������ҺΪǿ�������Σ�̼������Ӳ���ˮ�⣬��Һ�ʼ��ԣ�
��2����Һ��������Ũ��Խ����Һ��pHԽС����Һ������������Ũ��Խ����Һ��pHԽ�ݴ˽����жϣ�
��3��������������ƶ���ǿ����ʣ�����Һ�������Ӻ�����������Ũ����ȣ����Һ��pH=7��������Һ�����ȣ�
��4���������Һ�������ϣ�����Ϊ��Ũ�ȵĴ����ƺ�̼�����ƣ�̼��������ӵ�ˮ��̶ȴ��ڴ�������ӵ�ˮ��̶ȣ���c��CH3COO-����c��HCO3-����
��5������������þ���ܶȻ�����Һ������������Ũ�ȼ����þ����Ũ�ȣ�
��� �⣺��1��N2CO3��ǿ�������Σ�ˮ���ˮ��Һ���ʼ��ԣ�ˮ�����ӷ���ʽΪ��CO32-+H2O=HCO3-+OH-��
�ʴ�Ϊ�����ԣ�CO32-+H2O=HCO3-+OH-��
��2����0.1mol/L CH3COOH��Һ��������Ũ��С��0.1mol/L����Һ��pH��1����pH=13NaOH��Һ����0.05mol/L H2SO4��Һ��������Ũ��Ϊ0.1mol/L����ҺpH=1����0.1mol/L Na2CO3��Һ�������ԣ���������Һ��pH�ɴ�СΪ���ڢܢ٢ۣ�
�ʴ�Ϊ���ڢܢ٢ۣ�
��3����pH=13NaOH��Һ������������Ũ��Ϊ0.1mol/L����0.05mol/L H2SO4��Һ��������Ũ��Ϊ0.1mol/L�����Һ��pH=7������������������ƶ���ǿ����ʣ�������Һ�����ȣ�����a��b=1��1��
�ʴ�Ϊ��1��1��
��4�����������Һ�������ϣ�������ҺΪ��Ũ�ȵĴ����ƺ�̼�����ƣ�̼��������ӵ�ˮ��̶ȴ��ڴ�������ӵ�ˮ��̶ȣ���c��CH3COO-����c��HCO3-������Һ������Ũ���ɴ�С��˳��Ϊ��c��Na+����c��CH3COO-����c��HCO3-����c��OH-����c��H+����c��CO32-����
�ʴ�Ϊ��c��Na+����c��CH3COO-����c��HCO3-����c��OH-����c��H+����c��CO32-����
��5������Һ���м�������NaOH���壬����pH=13����Һ������������Ũ��Ϊ0.1mol/L����þ�����Ѷ�Ϊ��c��Mg2+��=$\frac{5.6��1{0}^{-12}}{0��{1}^{2}}$mol/L=5.6��10-10mol/L��
�ʴ�Ϊ��5.6��10-10mol/L��
���� ���⿼��������ϵĶ����жϼ���ҺpH�ļ��㣬��Ŀ�Ѷ��еȣ��漰������ʵĵ���ƽ�⡢����ϵĶ����жϡ���ҺpH�ļ��㡢�������ܽ�ƽ��ļ����֪ʶ������֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ���ѧ���ķ������������������Ӧ��������ע����������Ũ�ȶ��Գ��÷�������ȷ����ϵĶ����жϷ�������ҺpH�ļ��㷽����

| A�� | ����ʹ����ˮ���ռ����� | |
| B�� | Na2O2�Ⱥ������Ӽ����ֺ��зǼ��Լ� | |
| C�� | ����Ԫ�ش�����̬��Ϊ����̬һ�������� | |
| D�� | Na2CO3������Һ�г���ͨ��CO2�����й������� |
| A�� | ţ�͡���ά�غ͵����ʶ�����Ȼ�߷��ӻ����� | |
| B�� | ����ˮ������ղ����������� | |
| C�� | �������ܷ���������Ӧ��ˮ�ⷴӦ | |
| D�� | Ũ���ὦ��Ƥ���ϣ�ʹƤ���ʻ�ɫ������Ũ����͵����ʷ�������ɫ��Ӧ |
| A�� | ������ˮ��ֻ��Cl2��H2O���ַ��� | |
| B�� | ������ˮ��ʹ��ɫʯ����Һ�ȱ�����ɫ | |
| C�� | ���õ���ˮpH����������ǿ | |
| D�� | ��ˮ�������������ݳ�����������Cl2 |
| A�� | Ԫ�����ڱ���8������ | B�� | 0��ԭ�ӵ�������������Ϊ8 | ||
| C�� | ��A���Ԫ��ȫ�ǽ���Ԫ�� | D�� | ��������ָ��1��2��3���� |
| A�� | ��ˮ | B�� | �������� | C�� | ���Ը������ | D�� | ���� |
| A�� | Fe ���� | B�� | Cu ���� | ||
| C�� | �����Ϸ���������Ӧ | D�� | �����Ϸ���������Ӧ |
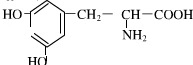 ��
��