��Ŀ����
9����һ���������Ļ����Һ��ȡ��20mL����������BaCl2��Һ�������ˡ�ϴ�ӡ���ɺ�ó���9.32g����Һ��4mol/L�Ŀ�������Һ35mLǡ����ȫ�кͣ�������1��ԭ�����Һ���������������ʵ���Ũ�ȣ�
��2����ȡ10mLԭ��Һ������0.96gͭ�۹��ȣ�����һ�����������Ϊ���٣���S��T��P����
��3����ȡ10mLԭ��Һ������1.92gͭ�۹��ȣ�����һ�����������Ϊ���٣���S��T��P����
��4����3����Ӧ�����Һ�м�����ٺ���1mol/L������ܽ�1.92gͭǡ����ȫ�ܽ⣿
���� ��1������H2SO4+BaCl2�T2HCl+BaSO4��������9.32gΪ���ᱵ������������n=$\frac{m}{M}$�������ᱵ�����ʵ��������ݷ���ʽ������������ʵ������ٸ���c=$\frac{n}{V}$�������Ũ�ȣ�
��Һ�����������ʵ������䣬���������Ʒ�Ӧ�����ӷ���ʽΪH++OH-�TH2O������NaOH�����ʵ����������ܵ����ʵ���������������������ʵ������ٸ���c=$\frac{n}{V}$�������Ũ�ȣ�
��2����3������Ϊ3Cu+8H++2NO3-�T3Cu2++4H2O+2NO�������ݷ�Ӧ�����ӷ���ʽ�����жϷ�Ӧ�Ĺ������⣬���ݲ����������ʼ�������NO�����ʵ������ٸ���V=nVm���㣻
��4�����ݣ�3����ȱ�������ӵ����ʵ������㽫1.92gͭǡ����ȫ�ܽ���Ҫ����1mol/L������������
��� �⣺��1������9.32gΪ���ᱵ������������H2SO4+BaCl2�T2HCl+BaSO4����
��֪n��H2SO4��=n��BaSO4��=$\frac{9.32g}{233g/mol}$=0.04mol��
��ԭ��Һ��c��H2SO4��=$\frac{0.04mol}{0.02L}$=2mol/L��
��Һ�����������ʵ������䣬��4.0mol•L-1NaOH��Һ��Ӧ����ȥ35mL��Һʱǡ����ȫ�кͣ���H++OH-�TH2O��
��֪n��H+��=n��OH-��=0.035L��4mol/L=0.14mol����Һ��������Դ�����ᡢ����ĵ��룬
��n��HNO3��+2��0.04mol=0.14mol��
���n��HNO3��=0.06mol��
��ԭ��Һ��c��HNO3��=$\frac{0.06mol}{0.02L}$=3mol/L��
�𣺻��Һ��H2SO4�����ʵ���Ũ��Ϊ2mol/L��HNO3�����ʵ���Ũ����3mol/L��
��2��0.96gͭ�����ʵ���Ϊ��$\frac{0.96g}{64g/mol}$=0.015mol��10mL�Ļ����Һ�к�������0.02mol��������0.03mol�������ӵ����ʵ�����0.07mol�������ͭ��Ӧ�����ӷ���ʽΪ��
3Cu+8H++2NO3-�T3Cu2++4H2O+2NO��
3mol 8mol 2mol
0.015mol 0.04mol 0.01mol
������������������ǹ����ģ�Cu���㣬��ȫ��Ӧ����������NO�����ʵ�����0.015mol��$\frac{2}{3}$=0.01mol�����״���µ������0.01mol��22.4L/mol=0.224L��
�𣺱�����������������Ϊ0.224L��
��3�����ݣ�2����֪��1.92gͭ�����ʵ���Ϊ0.03mol��10mL�Ļ����Һ�к�������0.02mol��������0.03mol�������ӵ����ʵ�����0.07mol�������ͭ��Ӧ�����ӷ���ʽΪ��
3Cu+8H++2NO3-�T3Cu2++4H2O+2NO��
3mol 8mol 2mol
0.03mol 0.08mol 0.02mol
������������ӹ����������Ӳ��㣬��������NO�����ʵ����ǣ�0.07mol��$\frac{1}{4}$=0.0175mol�����״���µ�����ǣ�0.175mol��22.4L/mol=0.392L��
�𣺱�����������������Ϊ0.392L��
��4�����ݣ�3����֪�������ӻ�ȱ�ٵ����ʵ���Ϊ��0.08mol-0.07mol=0.01mol����Ҫ1mol/L��������Һ���Ϊ��$\frac{0.01mol}{1mol/L��2}$=0.005L=5mL��
����3����Ӧ�����Һ�м���5����1mol/L������ܽ�1.92gͭǡ����ȫ�ܽ⣮
���� ���⿼��������㡢���ӷ�Ӧ�ļ��㣬��Ŀ�Ѷ��еȣ���ȷ������Ӧʵ��Ϊ���ؼ���ע�������ͭ����Ӧ�������ṩ�����ӣ�ֻҪ��������������������п��ܱ���ȫ��ԭ��ע�������������ӷ���ʽ���м���ķ�����

 �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д�| A�� | ���ʯ��ʯī | B�� | D2��H2 | ||
| C�� | CO��CO2 | D�� | ${\;}_{17}^{35}$Cl��${\;}_{17}^{37}$Cl |
| A�� | ����±�������Ƿ����ȣ��ɽ�����NaOH��Һ���Ⱥ�μ�AgNO3��Һ���� | |
| B�� | ������ӵ�Դ���������������Ѹ�բ������ӵ�Դ���������ӾͿ��Ա����� | |
| C�� | ���Ҵ���Ũ���������ϼ��ȿ����Ʊ������� | |
| D�� | ����������Ӧʵ����Թ��ڱڸ���������ϡ��ˮ����ϴȥ |
| A�� | �������뻹ԭ�������ʵ���֮��Ϊ1��5 | |
| B�� | ÿ��1 mol Cu�μӷ�Ӧʱת��2 mol e- | |
| C�� | ��������ֻ��CuSO4 | |
| D�� | Ũ����������������ǻ�ԭ�� |
| A�� | Cu+ϡHNO3 | B�� | Cu$\stackrel{��������}{��}$CuO$\stackrel{����}{��}$Cu��NO3��2 | ||
| C�� | Cu+ŨHNO3 | D�� | Cu$\stackrel{Cl_{2}}{��}$CuCl2$\stackrel{AgNO_{3}}{��}$Cu��NO3��2 |
| A�� | ʯ��ˮ | B�� | ���� | C�� | ���� | D�� | ˮ |
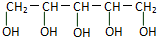 �������й�ľ�Ǵ��������в���ȷ���ǣ�������
�������й�ľ�Ǵ��������в���ȷ���ǣ�������| A�� | 1molľ�Ǵ��������Ʒ�Ӧ���ɲ���2.5molH2 | |
| B�� | ľ�Ǵ���һ�ֵ��ǣ����ܷ���ˮ�ⷴӦ | |
| C�� | ľ�Ǵ����ܽ���ˮ���ܷ���������Ӧ | |
| D�� | �����߿���ʳ�� |

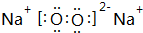 ��G�Ļ�ѧʽΪFe��OH��3��
��G�Ļ�ѧʽΪFe��OH��3��