��Ŀ����
����Ŀ��UO2���˵���������Ҫ�ĺ�ȼ�ϣ���֪��3(NH4)4[UO2(CO3)3]![]() 3UO2+10NH3��+9CO2��+N2��+9H2O��
3UO2+10NH3��+9CO2��+N2��+9H2O��
�ش��������⣺
(1)��̬��ԭ�Ӽ۵����Ų�ͼΪ______��
(2)��Ӧ������̬�����������ڷǼ��Է��ӵ���_______(�ѧʽ)��
(3)ij���˵�����ľ���ṹ��NaCl�͡�NaCl��Bom-Haberѭ����ͼ��ʾ����֪��Ԫ�ص�һ����̬ԭ�ӻ�õ��ӳ�Ϊ��̬������ʱ���ų���������Ϊ�������ܡ������й�˵����ȷ����________(����)��
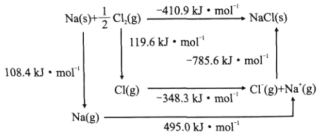
a.Cl-Cl���ļ���Ϊ119.6kJ/mol b.Na�ĵ�һ������Ϊ603.4kJ/mol
c.NaCl�ľ�����Ϊ785.6kJ/mol d.Cl�ĵ�һ��������Ϊ348.3kJ/mol
(4)����VSEPR�����Ʋ�CO32-�Ŀռ乹��Ϊ_________�������еĴ�آ�����÷���آ![]() ��ʾ������m���������γɴ�آ����ԭ������n���������γɴ�آ���ĵ�����(�籽�����еĴ�آ���ɱ�ʾΪآ
��ʾ������m���������γɴ�آ����ԭ������n���������γɴ�آ���ĵ�����(�籽�����еĴ�آ���ɱ�ʾΪآ![]() )����CO32-�еĴ�آ��Ӧ��ʾΪ_____
)����CO32-�еĴ�آ��Ӧ��ʾΪ_____
(5)UO2�������Ʊ�UF4��2UO2+5NH4HF2![]() 2UF4��2NH4F+3NH3��+4H2O������HF2�Ľṹ��ʾΪ[F��H��F]-����Ӧ�ж��ѵĻ�ѧ����_______ (����)��
2UF4��2NH4F+3NH3��+4H2O������HF2�Ľṹ��ʾΪ[F��H��F]-����Ӧ�ж��ѵĻ�ѧ����_______ (����)��
a.��� b.���Լ� c.���Ӽ� d.������ e.�Ǽ��Լ�
(6)�˵������ij���־�����ͼ��ʾ��
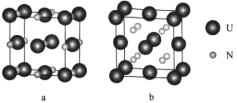
�پ���a����Ԫ�صĻ��ϼ�Ϊ__________����U��������������U��_______����
����֪����b���ܶ�Ϊdg/cm3��Uԭ�ӵİ뾶Ϊr1cm��Nԭ�ӵİ뾶ΪΪr2cm����NAΪ�����ӵ�������ֵ����þ����Ŀռ�������Ϊ___________(�г�����ʽ)��
���𰸡�![]() CO2 c��d ƽ��������
CO2 c��d ƽ�������� ![]() b��c +3 12
b��c +3 12 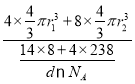 ��100%
��100%
��������
(1)�ȸ��ݹ���ԭ������дN�ĺ�������Ų�ʽ��Ȼ��ɸ��ݸ����ܼ����еĹ������������������ӹ��ɣ��õ���̬��ԭ�Ӽ۵����Ų�ͼ��
(2)��Ӧ������̬�����������NH3��CO2��N2��H2O�����ݷ����Ƿ�Գ��ж��Ƿ�Ϊ�Ǽ��Է��ӣ�
(3)����ͼʾ�������仯��ϻ�ѧ������������жϣ�
(4)���ݼ۲���ӶԻ������۷����жϣ�Cԭ�ӡ�Oԭ����ƽ�е�p������۵�������Ϊ4+2+6��3=24����������=24-2��3-4��3=6��Ϊ4ԭ�ӡ�6�����γɵĴ�������
(5) NH4HF2Ϊ���Ӿ��壬�������Ӽ���HF2-����������ۼ���NH4+�к�����λ�����ۼ���
(6)���þ�̯��������a��U��Nԭ�Ӹ����ȣ�Ȼ�����NԪ�ػ��ϼ۷���UԪ�ػ��ϼۣ��ھ�������U��������������Uԭ���ھ������ģ�Ȼ�����ͨ��һ��Uԭ�ӵľ��������غ���Ŀ����������Ŀ��
���ȼ���1�������к��е�U��Nԭ����Ŀ��Ȼ����㾧���������һ�������к��е�U��Nԭ����Ŀ���������������ԭ�����ռ������İٷֱȿɵ���ռ������ʡ�
(1)N��7��Ԫ�أ����ݹ���ԭ�����ɵ����������Ų�ʽ1s22s22p3����۵��ӵĹ������ʽΪ![]() ��
��
(2)��Ӧ������̬�����������NH3��CO2��N2��H2O��NH3��H2O�Ŀռ����в��Գƣ�������ɵ����IJ��غϣ����ڼ��Է��ӣ�CO2��N2�Ŀռ����жԳƣ�������������������غϣ��ǷǼ��Է��ӣ�����CO2�ǷǼ��Ի�������ӣ�
(3) a.Cl-Cl���ļ���Ϊ2��119.6kJ/mol=239.2kJ/mol��a����
b.Na�ĵ�һ������Ϊ495.0kJ/mol��b����
c.NaCl�ľ�����Ϊ785.6kJ/mol��c��ȷ��
d.Cl�ĵ�һ��������Ϊ348.3kJ/mol��d��ȷ��
�ʺ���ѡ����cd��
(4) CO32-�ļ۲���Ӷ���Ϊ3+![]() =3�����CO32-�ռ乹��Ϊƽ�������Σ�
=3�����CO32-�ռ乹��Ϊƽ�������Σ�
Cԭ�ӡ�Oԭ����ƽ�е�p������۵�������Ϊ4+2+6��3=24����������=24-2��3-4��3=6��Ϊ4ԭ�ӡ�6�����γɵĴ�������������Ϊ![]() ��
��
(5) NH4HF2Ϊ���Ӿ��壬�������Ӽ���HF2-����������ۼ���NH4+�к�����λ�����ۼ�������NH4HF2�������������У�a��b��c��d����Ӧ�ж��ѵĻ�ѧ����b��c��
(6)�پ���a��Uԭ�Ӹ���8��![]() +6��
+6��![]() =4������Nԭ�Ӹ���Ϊ��12��
=4������Nԭ�Ӹ���Ϊ��12��![]() +1=4�����һ�������к���U��Nԭ�Ӹ�����Ϊ4������ѧʽΪUN������Nԭ���������5�����ӣ�N���ϼ�Ϊ-3�ۣ�����UԪ�صĻ��ϼ�Ϊ+3������ͼʾ��֪���ھ�����U��������������U��һ�������������ϣ�ͨ��һ��Uԭ����8����������һ����������3��U��������������ÿ��Uԭ���ظ������Σ�����ھ�����U��������������Uԭ����Ϊ8��3��
+1=4�����һ�������к���U��Nԭ�Ӹ�����Ϊ4������ѧʽΪUN������Nԭ���������5�����ӣ�N���ϼ�Ϊ-3�ۣ�����UԪ�صĻ��ϼ�Ϊ+3������ͼʾ��֪���ھ�����U��������������U��һ�������������ϣ�ͨ��һ��Uԭ����8����������һ����������3��U��������������ÿ��Uԭ���ظ������Σ�����ھ�����U��������������Uԭ����Ϊ8��3��![]() =12��
=12��
����һ�������к���Uԭ����ĿΪU��8��![]() +6��
+6��![]() =4�����е�Nԭ����ĿΪ8��1=8��һ�������к��е�U��Nԭ�ӵ������ΪV(U)+V(N)=(4��
=4�����е�Nԭ����ĿΪ8��1=8��һ�������к��е�U��Nԭ�ӵ������ΪV(U)+V(N)=(4��![]() +8��
+8��![]() )cm3������ͼʾ��֪��������Ϊ4��Uԭ�ӵİ뾶��
)cm3������ͼʾ��֪��������Ϊ4��Uԭ�ӵİ뾶��![]() ����
����![]() L=4r1cm�����������V(����)=
L=4r1cm�����������V(����)=![]() ����þ�����ԭ��������Ϊ
����þ�����ԭ��������Ϊ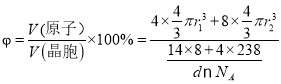 ��100%��
��100%��

 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�