��Ŀ����
����Ŀ��I.H2A��ˮ�д�������ƽ�⣺H2A![]() H+ ��HA- ��HA��
H+ ��HA- ��HA��![]() H+��A2- ��
H+��A2- ��
��1��NaHA��Һ�����ԣ�����Һ������Ũ�ȵĴ�С˳��Ϊ____________________��
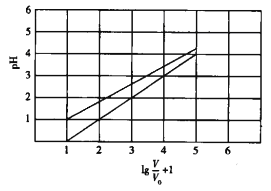
��2������ʱ������0.1 mol/L��NaHA��Һ����εμ�0.1mol/L KOH��Һ����Һ�����ԡ���ʱ�û����Һ�����й�ϵ�У�һ����ȷ����_______________��
A��c(Na+ )��c(K+) B��c(H +)c(OH)��1��10-14
C��c(Na+ )��c(K+) D��c(Na+ )��c(K+ )��c(HA��)��c(A2- )
��3����֪������H2A�ĸ���(CaA)������Һ�д�������ƽ����CaA(s)![]() Ca2+ (aq)��A2- (aq)���μ�����Na2A������c(Ca2+ )_______________������������������С����������������ԭ����________________��
Ca2+ (aq)��A2- (aq)���μ�����Na2A������c(Ca2+ )_______________������������������С����������������ԭ����________________��
��.����Cr2O72-�ķ�ˮ���Խϴ���ij������ˮ�к�4.00��10-3 mol/L��Cr2O72����Ϊʹ��ˮ�ܴ���ŷţ������´�����![]()
��1���÷�ˮ�м���FeSO4��7H2O��ϡ���ᣬ������Ӧ�����ӷ���ʽΪ��_______________��
��2����ʹ25 L�÷�ˮ��Cr2O7 ת��ΪCr3+����������Ҫ����__________g FeSO4��7H2O��
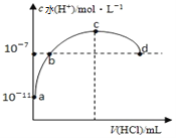
��3����������ķ�ˮ�в�����c(Fe)��1��10-13mol/L��������� Cr3+ ��Ũ��Ϊ__________������֪��Ksp[Fe(OH)3]��1.0��10-38mol/L��Ksp[Cr(OH)3]��1.0��10-31 mol/L ��
���𰸡�I.��1��c(Na��)��c(HA��)��c(H��)��c(A2��)��c(OH��) ��2��AB
��3����С������Na2A������c(A2- )���Ӷ������ܽ�ƽ�����ƣ�c(Ca2+ )��С
��.��1��Cr2O72����6Fe2����14H����2Cr3����6Fe3����7H2O ��2��166.8 ��3��1.0��10��6mol/L
��������
����I.��1��H2A��ˮ�д�������ƽ����H2A![]() H+ ��HA- ��HA��
H+ ��HA- ��HA��![]() H+��A2- ������NaHAֻ���ڵ���ƽ������Һ����������Һ������Ũ�ȵĴ�С˳��Ϊc(Na��)��c(HA��)��c(H��)��c(A2��)��c(OH��)��
H+��A2- ������NaHAֻ���ڵ���ƽ������Һ����������Һ������Ũ�ȵĴ�С˳��Ϊc(Na��)��c(HA��)��c(H��)��c(A2��)��c(OH��)��
��2��A��NaHA��Һ�����ԣ���0.1mol/L��NaHA��Һ����εμ�0.1mol/L KOH��Һ����Һ������ʱ��NaHA�����ʵ���Ӧ�����������ص����ʵ���������ͬһ�����Һ��c(Na+)��c(K+)��A��ȷ��B��ˮ�����ӻ��������¶��йأ��¶�Խ�ߣ�ˮ�����ӻ�����Խ��������ˮ�����ӻ�������10��14��B��ȷ��C������A�з�����֪C����D�����ݵ���غ��֪c(Na+)��c(K+)��c(H+)��c(HA��)��2c(A2- )����c(H��)��c(OH��)������c��Na+��+c��K+��=c��HA-��+2c��A2-����D����ѡAB��
��3�����ڼ���Na2A������c(A2- )���Ӷ������ܽ�ƽ�����ƣ�c(Ca2+ )��С��
��.��1���������ӱ����������ӷ���ʽΪCr2O72����6Fe2����14H����2Cr3����6Fe3����7H2O��
��2��ij������ˮ�к�4.00��10-3 molL-1��Cr2O72����n��Cr2O72������25L��4.00��10-3mol/L��0.1mol������������ԭ��Ӧ���ӷ���ʽCr2O72����6Fe2����14H����2Cr3����6Fe3����7H2O���õ�n��Fe2+����0.6mol����ҪFeSO47H2O������=0.6mol��278g/mol��166.8g��
��3����������ķ�ˮ�в�����c��Fe3+����1��10-13molL-1��Ksp[Fe��OH��3]��c��Fe3+����c3��OH-����1.0��10-38������õ�c3��OH-����1��10-25mol/L���������Cr3+��Ũ��ΪKsp[Cr��OH��3]��c��Cr3+��c3��OH-����1.0��10-31 ��c��Cr3+����1��10-6molL-1��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�