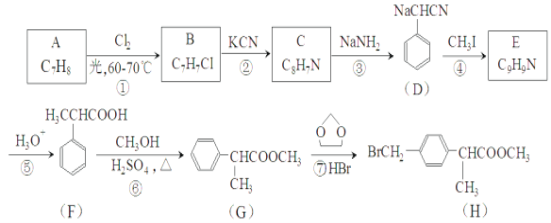
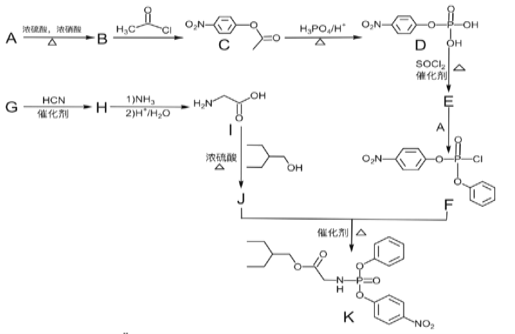
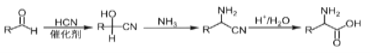
��Ŀ����
����Ŀ������ϡ���з����ߵ�Ԫ�أ����ڵ��Ӳ��ϡ������ȷ����Ӧ�ù㷺�����Է�̼���(��CeFCO3��BaO��SiO2��)Ϊԭ���Ʊ�������(CeO2)�����ⶨ�䴿�ȡ��乤���������£�

��֪����ϡ��������SO![]() �γɸ��γ�����
�γɸ��γ�����
Ce2(SO4)3��Na2SO4��nH2O==== Ce2(SO4)3��Na2SO4��nH2O��(�����)��
�����壺һ���л���ṹ��ʽΪ![]() �������������ױ�����Ϊ(SCN2H3)2��
�������������ױ�����Ϊ(SCN2H3)2��
��Ce3���ڿ������ױ�����ΪCe4����
�ش��������⣺
(1)����ʱ��Ϊ����߱���Ч�ʣ��ɲ�ȡ�Ĵ�ʩ��________________________________________
(2)CeFCO3��CeԪ�صĻ��ϼ�Ϊ___________������A����Ҫ�ɷ���_____________
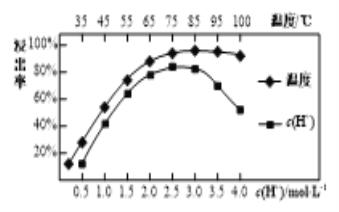
(3)���պ�������������ϡ���Ľ����ʺ�����Ũ�ȡ��¶��йأ���ͼ��ʾ��Ӧѡ������˵�����Ϊ___________������Ũ�ȹ���ʱ�������ʼ�С��ԭ����__________��
(4)���������Ŀ���ǽ�Ce4����ԭΪCe3������Ӧ�Ļ�ѧ����ʽΪ_________��
(5)����ۼ��������ͨ���������H2O2������ҪĿ��Ϊ_________��
(6)����ܵ����ӷ���ʽΪ________________________��
(7)ȡ���ò�ƷCeO2 8.0g����30 mL�������20 mL������Һ�����ܽ⣬��ȴ�����º����250 mL��Һ��ȡ25.00 mL��Һ��0.2000 mol��L��1���������[(NH4)2Fe(SO4)2]��Һ�ζ�����֪�ζ�ʱ�����ķ�ӦΪFe2����Ce4��==== Fe3����Ce3�����ﵽ�ζ��յ�ʱ���������������Һ20.50 mL����ò�Ʒ�Ĵ���Ϊ____________��
���𰸡�����̼������������Ӵ�������ӳ�����ʱ�� ��3�� BaSO4��SiO2 �¶�85����c(H��)2.5mol/L ��Һ��c(SO![]() )������ϡ�������γɸ��γ�����ʹ�����ʽ��� 2Ce(SO4)2��2
)������ϡ�������γɸ��γ�����ʹ�����ʽ��� 2Ce(SO4)2��2![]() ==== Ce2(SO4)3��(SCN2H3)2��H2SO4 ��ֹCe3�������� 2Ce3����6HCO
==== Ce2(SO4)3��(SCN2H3)2��H2SO4 ��ֹCe3�������� 2Ce3����6HCO![]() ==== Ce2(CO3)3����3CO2����3H2O 88.15%
==== Ce2(CO3)3����3CO2����3H2O 88.15%
��������
��̼���(��CeFCO3��BaO��SiO2��)�ڿ����б��գ�CeԪ�ر��������õ�CeO2��CeF4��֮����ϡ�����ȡ���õ�������AΪ������BaSO4�Լ�SiO2����ҺA����Ҫ����Ce4+��F���ȣ���̼�������������ƣ�������Ŀ��֪��Ϣ��֪CeԪ��ת��ΪCe2(SO4)3��Na2SO4��nH2O���������μ���NaOH��Һ��ϡ���ᣬ�õ���Ce3+����Һ��Ȼ������Һ�м���̼����泥�̼������������̼�����Ce3+��ϵõ�Ce2(CO3)3�������������յõ�CeO2��
(1)����̼������������Ӵ�������ӳ�����ʱ�䶼������߱���Ч�ʣ�
(2)CeFCO3��FΪ-1�ۣ�OΪ-2�ۣ�CΪ+4�ۣ�����CeΪ+3�ۣ�����A��ҪΪ������BaSO4��SiO2��
(3)��ͼ��֪��c(H��)=2.5mol/L��������ߣ��¶�Ϊ85��ʱ��������ߣ��������˵�����Ϊ�¶�85����c(H��)=2.5mol/L��������Ŀ��֪��Ϣ��֪��Һ��c(SO![]() )������ϡ�������γɸ��γ�����ʹ�����ʽ��ͣ�
)������ϡ�������γɸ��γ�����ʹ�����ʽ��ͣ�
(4)������Ŀ��Ϣ��֪��Ӧ������![]() ��������(SCN2H3)2����Ce4������ԭΪCe3�������ݵ����غ�ɵû�ѧ����ʽΪ2Ce(SO4)2��2
��������(SCN2H3)2����Ce4������ԭΪCe3�������ݵ����غ�ɵû�ѧ����ʽΪ2Ce(SO4)2��2![]() ===Ce2(SO4)3��(SCN2H3)2��H2SO4��
===Ce2(SO4)3��(SCN2H3)2��H2SO4��
(5)����˫��ˮ���Է�ֹCe3����������
(6)��Ce3+��Һ�м���̼�������Һ��̼������������̼�����Ce3+������ɳ������ٽ�̼������ĵ��룬���Ի�������������ӣ��������ֺ�̼�����������ɶ�����̼��ˮ���������ӷ���ʽΪ2Ce3����6HCO![]() ===Ce2(CO3)3����3CO2����3H2O��
===Ce2(CO3)3����3CO2����3H2O��
(7)���ݵζ�ʱ�����ķ�Ӧ��֪25.00mL��Һ��n(Ce4+)=n{[(NH4)2Fe(SO4)2]}=0.2000mol/L��0.0205L=0.0041mol������Ʒ��n(Ce4+)=0.0041mol��![]() =0.041mol��n(CeO2)=n(Ce4+)=0.041mol��������Ʒ�Ĵ���Ϊ
=0.041mol��n(CeO2)=n(Ce4+)=0.041mol��������Ʒ�Ĵ���Ϊ![]() 100%=88.15%��
100%=88.15%��

����Ŀ����1���Ҵ���δ����ȼ������ѡ������Һ��ȼ�ϡ�2.0g�Ҵ���ȫȼ������Һ̬ˮ�ų�59.43kJ�����������Ҵ�ȼ�յ��Ȼ�ѧ����ʽ__��
��2����֪�����Ȼ�ѧ����ʽ��
��C(s��ʯī)+O2(g)�TCO2(g) ��H=-393.5kJmol-1
��2H2(g)+O2(g)�T2H2O(l) ��H=-571.6kJmol-1
��2C2H2(g)+5O2(g)�T4CO2(g)+2H2O(l) ��H=-2599kJmol-1
��д��C(s��ʯī)��H2(g)����1mol C2H2(g)���Ȼ�ѧ����ʽ_____��
��3����֪���ֹ��ۼ��ļ����������±���
���ۼ� | N��N | H��H | N��H |
����(kJ/mol) | 946 | 436 | 390.8 |
д���ϳɰ���Ӧ���Ȼ�ѧ����ʽ��____��