��Ŀ����
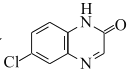
����Ŀ��ij����ɫ����A����������Ԫ�أ�ʽ��С��160����������ҩ��Ϊ̽������ɺ����ʣ���Ʋ��������ʵ�飺

��֪�����������������漰�ķ�Ӧ����ֽ��С�
��B��C��D��Ϊ�������塣��ͬ���������B���ܶȴ�����C��
�ۺ�ɫ����![]() ���������μ�KSCN������������������ˮ����Һ��졣
���������μ�KSCN������������������ˮ����Һ��졣
���ʴ�
��1��д������B�ĵ���ʽ__������A�Ļ�ѧʽ��__��
��2����ɫ����E������Ũ���ᷴӦ�����ӷ�Ӧ����ʽ��__��
���𰸡�![]() FeC2O4 3FeO + 10H+ + NO3�� = NO + 3Fe3+ + 5H2O
FeC2O4 3FeO + 10H+ + NO3�� = NO + 3Fe3+ + 5H2O
��������
B��C��D��Ϊ��������˵��D����Ϊ���������ʵ���Ϊ![]() ����ͬ���������B���ܶȴ�����C��˵��BΪ������̼��B��������Ʒ�Ӧ����CΪһ����̼���������ʵ���Ϊ
����ͬ���������B���ܶȴ�����C��˵��BΪ������̼��B��������Ʒ�Ӧ����CΪһ����̼���������ʵ���Ϊ![]() ��������0.1mol����������2CO2 + 2Na2O2 = 2Na2CO3 + O2����˶�����̼�����ʵ���Ϊ0.2mol��CO���ʵ���Ϊ0.2mol����ɫ����E���������μ�KSCN������������˵���������ӣ���������ˮ����Һ��죬˵��ԭ����ΪFeO����m(FeO) = 28.8g0.2mol��28 gmol1 0.2mol��44 gmol1 = 14.4g����FeO���ʵ���
��������0.1mol����������2CO2 + 2Na2O2 = 2Na2CO3 + O2����˶�����̼�����ʵ���Ϊ0.2mol��CO���ʵ���Ϊ0.2mol����ɫ����E���������μ�KSCN������������˵���������ӣ���������ˮ����Һ��죬˵��ԭ����ΪFeO����m(FeO) = 28.8g0.2mol��28 gmol1 0.2mol��44 gmol1 = 14.4g����FeO���ʵ���![]() �����n(Fe): n(C): n(O) = 0.2mol:(0.2mol+0.2mol):(0.2mol+0.2mol+0.2mol��2) =1:2:4����ѧʽΪFeC2O4��
�����n(Fe): n(C): n(O) = 0.2mol:(0.2mol+0.2mol):(0.2mol+0.2mol+0.2mol��2) =1:2:4����ѧʽΪFeC2O4��
������BΪ������̼�������ʽ![]() ��������������õ�����A�Ļ�ѧʽ��FeC2O4���ʴ�Ϊ��
��������������õ�����A�Ļ�ѧʽ��FeC2O4���ʴ�Ϊ��![]() ��FeC2O4��
��FeC2O4��
�ƺ�ɫ����E��FeO������Ũ���ᷴӦ������������һ��������ˮ�������ӷ�Ӧ����ʽ��3FeO + 10H+ + NO3�� = NO + 3Fe3+ + 5H2O���ʴ�Ϊ��3FeO + 10H+ + NO3�� = NO + 3Fe3+ + 5H2O��

 �Ķ��쳵ϵ�д�
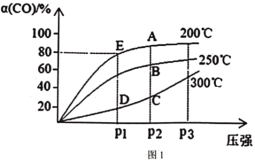
�Ķ��쳵ϵ�д�����Ŀ����10L�����ܱ������г���X(g)��Y(g)��������ӦX(g)+Y(g)![]() M(g)+N(g)������ʵ���������±���
M(g)+N(g)������ʵ���������±���
ʵ���� | �¶�/�� | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | |
n(X) | n(Y) | n(M) | ||
�� | 700 | 0.40 | 0.10 | 0.090 |
�� | 800 | 0.10 | 0.40 | 0.080 |
�� | 800 | 0.20 | 0.30 | a |
�� | 900 | 0.10 | 0.15 | b |
����˵������ȷ���ǣ� ��
A.ʵ����У���5minδ���n(M)=0.050mol����0��5minʱ���ڣ���N��ʾ��ƽ����Ӧ��v(N)=1.0��10-3mol��L-1��min-1
B.ʵ����У��ﵽƽ��ʱ��Y��ת����Ϊ20%
C.ʵ����У��÷�Ӧ��ƽ�ⳣ��K=1.0
D.ʵ����У��ﵽƽ��ʱ�� b>0.060
����Ŀ��25��ʱ������ƽ�ⳣ����
��ѧʽ | CH3COOH | H2CO3 | HClO |
����ƽ�ⳣ�� | 1.8��10-5 | K1=4.3��10-7 K2=5.6��10-11 | 3.0��10-8 |
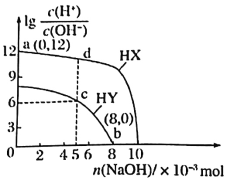
��1��������ˮϡ��0.10mol/L�Ĵ��ᣬ�����и�ʽ��ʾ����ֵ��ˮ�������Ӷ��������_____��
A.![]() B.
B.![]() C.
C.![]() D.
D.![]()
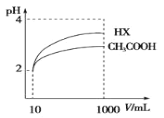
��2�����Ϊ10mLpH=2�Ĵ�����Һ��һԪ��HX�ֱ��ˮϡ����1000mL��ϡ����pH�仯��ͼ����HX�ĵ���ƽ�ⳣ��______(������������������������С����)�����ƽ�ⳣ����ϡ�ͺ�HX��Һ��ˮ���������c(H+)_____������Һ��ˮ���������c(H+)(������������������������С����)��