��Ŀ����
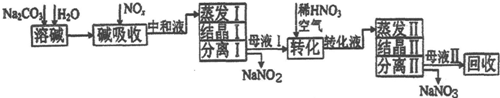
5������þ[Mg��ClO3��2]����������������ݼ��ȣ�ʵ�����Ʊ�����Mg��ClO3��2•6H2O��������ͼ1��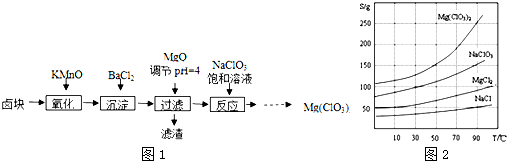
��֪����±����Ҫ�ɷ�ΪMgCl2•6H2O������MgSO4��FeCl2�����ʣ�
�����ֻ�������ܽ�ȣ�S�����¶ȣ�T���仯������ͼ2��ʾ��
��1����������Ҫ����Ҫ����������©�������������ձ���
��2������BaCl2��Ŀ���dz�ȥSO42-����MgO�����������������Ҫ�ɷ�ΪBaSO4��Fe��OH��3��
��3������NaClO3������Һ������Ӧ�Ļ�ѧ����ʽΪMgCl2+2NaClO3�TMg��ClO3��2+2NaCl�����ٽ�һ����ȡMg��ClO3��2•6H2O��ʵ�鲽������Ϊ���������ᾧ���ڳ��ȹ��ˣ�����ȴ�ᾧ���ܹ��ˡ�ϴ�ӣ�
��4����Ʒ��Mg��ClO3��2•6H2O�����IJⶨ��
����1��ȷ����3.50g��Ʒ���100mL��Һ��
����2��ȡ10.00mL����ƿ�У�����10.00mLϡ�����20.00mL 1.000mol•L-1��FeSO4��Һ���ȣ�
����3����ȴ�����£���0.100mol•L-1 K2Cr2O7��Һ�ζ�ʣ���Fe2+���յ㣬�˹����з�Ӧ�����ӷ���ʽΪ��Cr2O72-+6Fe2++14H+=2Cr3++6Fe3++7H2O��
����4��������2��3�ظ����Σ�ƽ������K2Cr2O7��Һ15.00mL��
��д������2�з�����Ӧ�����ӷ���ʽ��ClO3-+6Fe2++6H+=6Fe3++Cl-+3H2O��
�ڲ�Ʒ��Mg��ClO3��2•6H2O����������Ϊ78.3%��
���� ±��ijɷ���MgCl2•6H2O��MgSO4��FeCl2������˫��ˮ֮���������ӿ��Ա�����Ϊ���������ӣ����������м����Ȼ�����Һ�����Խ����������ת��Ϊ���ᱵ��������������þ������pH=4�����Դٽ������ӵ�ˮ�⣬��������ת��Ϊ������������ȥ�����ˣ��õ�����Һ���Ȼ�þ�����Ը����ܽ�����¶ȵ�Ӱ����������Ҫ��ȡ�����ʣ�
��1������ʵ���õ���������©�������������ձ�����ֽ������̨�ȣ�
��2����Һ�ﺬ�������������Ҫ��ȥ����˵μӹ������Ȼ�����Һ�����ݼ�����þ����Һ��pHΪ4�����Գ������������ж���������Ҫ�ɷ֣�
��3������NaClO3������Һ�ᷢ�����·�Ӧ��MgCl2+NaClO3��Mg��ClO3��2+NaCl����δ��ƽ�����������ʵ��ܽ�ȴ�С����Һ��þ���ķ���������Ũ�������ȹ��ˣ���ȴ�ᾧ�����ˡ�ϴ�ӡ����
��4���ٲ���2����������Ӿ��������ԣ����Խ�������������Ϊ�����ۣ�
�ڸ��ݻ�ѧ��ӦClO3-+6Fe2++6H+=6Fe3++Cl-+3H2O�Լ�Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O�����Եó���ClO3-��6Fe2+��Cr2O72-��6Fe2+�����ݸ�������֮��Ĺ�ϵʽ�ɼ���ٷֺ�����
��� �⣺±��ijɷ���MgCl2•6H2O��MgSO4��FeCl2������˫��ˮ֮���������ӿ��Ա�����Ϊ���������ӣ����������м����Ȼ�����Һ�����Խ����������ת��Ϊ���ᱵ��������������þ������pH=4�����Դٽ������ӵ�ˮ�⣬��������ת��Ϊ������������ȥ�����ˣ��õ�����Һ���Ȼ�þ�����Ը����ܽ�����¶ȵ�Ӱ����������Ҫ��ȡ�����ʣ�
��1������ʵ���õ���������©�������������ձ�����ֽ������̨�ȣ����в��������У�©�������������ձ����ʴ�Ϊ��©�������������ձ���
��2���μ�BaCl2��Һ�Ὣ��Һ�����������SO42-��������˵μӵ�Ŀ�ľ��dz��ӣ���������þ��������Һ��pHΪ4����ʱ�������γ��˳��������������������Ѿ�����������ӷ�Ӧ���������ᱵ�������ʴ�Ϊ����ȥSO42-��BaSO4��Fe��OH��3��
��3������NaClO3������Һ�ᷢ�����·�Ӧ��MgCl2+2NaClO3�TMg��ClO3��2+2NaCl�����������ʵ��ܽ�ȴ�С����Һ��þ���ķ�����MgCl2+2NaClO3�TMg��ClO3��2+2NaCl��������Ũ�������ȹ��ˣ���ȴ�ᾧ�����ˡ�ϴ�ӡ����
�ʴ�Ϊ��MgCl2+2NaClO3�TMg��ClO3��2+2NaCl���������ȹ��ˣ���ȴ�ᾧ��
��4������������Ӿ��������ԣ����Խ�������������Ϊ�����ۣ����ӷ���ʽΪClO3-+6Fe2++6H+=6Fe3++Cl-+3H2O���ʴ�Ϊ��ClO3-+6Fe2++6H+=6Fe3++Cl-+3H2O��
�ڸ��ݻ�ѧ����ʽ��ClO3-+6Fe2++6H+=6Fe3++Cl-+3H2O�Լ�Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O�����Եó���ClO3-��6Fe2+��Cr2O72-��6Fe2+����0.100mol•L-1 K2Cr2O7��Һ�ζ����յ���̿��Եó�ʣ����������ӵ����ʵ���Ϊ��0.100mol•L-1��0.015L��6=0.009mol������������ӷ�Ӧ���������ӵ����ʵ���Ϊ��20��10-3L��1.000mol•L-1-0.009mol=0.011mol����������ӵ����ʵ���Ϊ$\frac{1}{6}$��0.011mol����Ʒ��Mg��ClO3��2•6H2O��������������$\frac{1}{2}$��0.011��299g/mol����10��$\frac{1}{3.5}$��100%=78.3%���ʴ�Ϊ��78.3%��
���� ���⿼��Գ�������Ԫ�ؼ��仯������Ҫ���ʵ����գ��Լ������ӷ�Ӧʵ�ʵ���ʶ��ͬʱ����Ӧ�û���֪ʶ�����ѧ����������Լ���ͼ���Ĺ۲졢���������������ܽ�ȸ����Ӧ�ã����������ķ������������ӵij���ԭ����Լ�ѡ����Ŀ�Ѷ��еȣ�

 �������ϵ�д�
�������ϵ�д�| ������ | ����� | ǿ����� | ������� | �ǵ���� | |
| A | ˮ���� | ���� | ���� | ������ | �ɱ� |
| B | �� | ��ˮ | ̼��� | ˮ | ���� |
| C | ��ˮ | Ư�� | ������ | ̼�� | �Ȼ��� |
| D | �մ� | ������ | �Ȼ��� | ������ | ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��ѧ��Ӧ�����м������ʱ仯���������仯���������ͷŻ����������ʱ仯Ϊ���� | |
| B�� | �о���ѧ��Ӧ���еķ����������ǻ�ѧ��Ӧԭ������Ҫ���� | |
| C�� | �������ˮ��Һ�еķ�Ӧ��Ӧ���ʺܸߣ�����Ϊ���෴Ӧ����Ҫ��������� | |
| D�� | ������չ��������㹤ҵ��չ�Ե����������Ͻ��ܼ��ŵ�Ҫ�� |
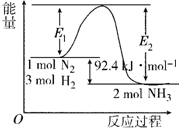 �´ﵽƽ��ʱH2��ת����Ϊ33.3%�����¶��µ�ƽ�ⳣ��K��ֵΪ$\frac{100}{27}$���������¶ȣ�Kֵ��С���������С�����䡱����
�´ﵽƽ��ʱH2��ת����Ϊ33.3%�����¶��µ�ƽ�ⳣ��K��ֵΪ$\frac{100}{27}$���������¶ȣ�Kֵ��С���������С�����䡱����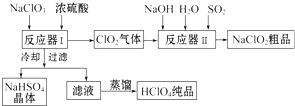 ��ҵ������������ʱ����ͬʱ����һ�ֳ�������Ҫ������������Ư���������ƣ�NaClO2���������������£�
��ҵ������������ʱ����ͬʱ����һ�ֳ�������Ҫ������������Ư���������ƣ�NaClO2���������������£�