��Ŀ����
9���жϺ�����ǿ����һ����������ǣ���������ӽṹ�к����ǻ���ԭ����Խ�࣬�ú����������Խǿ�����±���ʾ| ���� | ������ | ���� | ���� | ������ |
| �ṹʽ | Cl-OH | 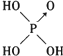 | 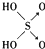 |  |
| ���ǻ���ԭ���� | 0 | 1 | 2 | 3 |
| ���� | ���� | ��ǿ�� | ǿ�� | ��ǿ�� |
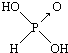 ��
�� ����������ǿ��ԭ�������Ậ��1�����ǻ���ԭ�ӣ���������û�з��ǻ���ԭ�ӣ��������������ǿ�������������
����������ǿ��ԭ�������Ậ��1�����ǻ���ԭ�ӣ���������û�з��ǻ���ԭ�ӣ��������������ǿ���������������2��������������������������������Һ��Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ����H3PO3+2NaOH=Na2HPO3+2H2O�� ��H3AsO3+3NaOH=Na3AsO3+3H2O��
���� ��1�����ݺ�������ӽṹ�к����ǻ���ԭ����Խ�࣬�ú����������Խǿ������
��2����ͼӦ�����κ�ˮ����ϼ�Ԫ�������
��� �⣺��1����������ӽṹ�к����ǻ���ԭ����Խ�࣬�ú����������Խǿ�������� ����1�����ǻ���ԭ�ӣ�����ǿ�ᣬ������
����1�����ǻ���ԭ�ӣ�����ǿ�ᣬ������ û�з��ǻ���ԭ�ӣ��������������������ԣ�
û�з��ǻ���ԭ�ӣ��������������������ԣ�
�ʴ�Ϊ�������Ậ��1�����ǻ���ԭ�ӣ���������û�з��ǻ���ԭ�ӣ��������������ǿ������������ԣ�
��2����ͼӦ�����κ�ˮ���������к���2���ǻ������ڶ�Ԫ�ᣬ������������Ԫ�ᣬ�������������ֱ���������Ƶķ�Ӧ����ʽΪ��H3PO3+2NaOH=Na2HPO3+2H2O��H3AsO3+3NaOH=Na3AsO3+3H2O��
�ʴ�Ϊ��H3PO3+2NaOH=Na2HPO3+2H2O��H3AsO3+3NaOH=Na3AsO3+3H2O��
���� ���⿼�����ʵ����ʼ���Ӧ����ʽ����д��Ϊ��Ƶ���㣬ע�����ϰ���е���Ϣ�����Ե��ж�Ϊ���Ĺؼ���������ѧ���ķ��������Ŀ��飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
19����Է�������Ϊ128���л���A��ȫȼ�պ�ֻ���ɶ�����̼��ˮ����A����һ����Ԫ̼��������NaHCO3��Һ��Ӧ���ɶ�����̼�����价�ϵ�һ������У�������
| A�� | 5�� | B�� | 4�� | C�� | 3�� | D�� | 2�� |
20������[��CN��2]���軯�ﶼ�Ǿ綾�����ʣ�����ӵĽṹʽΪN��C-C��N��������±�����ƣ���������������ǣ�������
| A�� | ���������ԭ�ӹ�ֱ�ߣ��ǷǼ��Է��� | |
| B�� | �������N���ӻ�����ΪSP | |
| C�� | ���������к��ЦҼ��ͦм� | |
| D�� | �������C��N��������C��C���� |
17�������£����ݻ�������ܱ������н�N2��H2��ϣ�ʹ����һ�������·�����Ӧ��N2��g��+3H2��g��?2NH3��g�������ﵽ��ѧƽ�⣬���ݱ������ݻش��й�����
��1������a=2.5��e=1.5
��2������Ӧ��3min�ﵽƽ�⣬��Ӧ����v��N2��=0.5mol•L-1•min-1��
��3���÷�Ӧ��ϵ����ʼ״̬��ƽ��״̬��ѹǿ֮��Ϊ17��11��
��4��������Ӧ�����У���������¶ȣ���Ӧ�ٶȼ�С�������С�������䡱����
��5�����и����У�����˵��������Ӧ�ﵽƽ�����BCD������ţ�
A�����������ܶȲ��ٱ仯
B����������ѹǿ���ٱ仯
C��N2������Ӧ���ʵ����淴Ӧ����
D��N2��Ũ�ȱ��ֺ㶨���䣮
| ���ʵ���Ũ�� | N2 | H2 | NH3 |
| ��ʼ/mol•L-1 | a | 6 | 0 |
| ת��/mol•L-1 | b | c | d |
| ƽ��/mol•L-1 | 1 | e | 3 |
��2������Ӧ��3min�ﵽƽ�⣬��Ӧ����v��N2��=0.5mol•L-1•min-1��
��3���÷�Ӧ��ϵ����ʼ״̬��ƽ��״̬��ѹǿ֮��Ϊ17��11��
��4��������Ӧ�����У���������¶ȣ���Ӧ�ٶȼ�С�������С�������䡱����
��5�����и����У�����˵��������Ӧ�ﵽƽ�����BCD������ţ�
A�����������ܶȲ��ٱ仯
B����������ѹǿ���ٱ仯
C��N2������Ӧ���ʵ����淴Ӧ����
D��N2��Ũ�ȱ��ֺ㶨���䣮
4����������������ǣ�������
| A�� | �ý����ƿ������Ҵ������� | |
| B�� | �ø������������Һ�����ּ����3-��ϩ | |
| C�� | ��ˮ�����ֱ����屽 | |
| D�� | ����ˮ�����Ը��������Һ���Լ���1-��ϩ����ȩ |
14������˵������ȷ���ǣ�������
| A�� | HCl��HBr��HI���۵�е������뷶�»�����С�й� | |
| B�� | H2O���۵�е����H2S��������H2O����֮�������� | |
| C�� | �Ҵ���ˮ���ܿ�������������ԭ������ | |
| D�� | ������ˮ���Ӽ䲻���γ�������ֻ�ѧ�� |
1������Σ��֮�£��й�����ҵ�ܵ��˾�ij����2009��6��30����7��2�����Ϻ��¹��ʲ������ľٰ���й���������ҵչ����ȴ����ˡ�չ����ҵ��Ȼ�������Ŀںţ���Ϊȫ����ҵ�Ľ�������㣮ij��ѧ��ȤС���ͬѧ�Ǿ��������仯��������ʱȽ�չ��̽�������������һЩʵ���������£�
��������������й�ʵ���������£�
��1������Ƭ�������ṯ��Һ�У���Ƭ����Ұ�����ʪ�����Ժ���Ƭ�ϸ�����Һ̬����ɫ�����ʣ�������Ƭ����һ��ʱ�䣬��Ƭ���泤����ɫ��״��ֳ���Ƭʱ����ɫ��״�����䣬��Ƭ���̣���ʵ���ɫ��״��������ϡ������������ų�����д��ֱ�ӵ�����Ƭ���̵��Ȼ�ѧ��Ӧ����ʽ4Al��s��+3O2��g��=2Al2O3��s������H=-2408KJ/mol��
��2������Լ6cm����2cm��ͭƬ����Ƭ��һƬ���ֱ��ý�����ƽ�еع̶���һ�����ϰ��ϣ����2cm������ͭƬ����Ƭ�ֱ�͵������ġ�+������-���������ӣ�������ָ������м�λ�ã�ȡ����50mL��С�ձ�����һ���ձ���ע��Լ40mL��Ũ���ᣬ����һֻ�ձ���ע��40mL0.5mol/L��������Һ���Իش��������⣺
�����缫ͬʱ����ϡ�����У�������ָ��ƫ�������������ͭ����������Ƭ�ϵ缫��ӦʽΪAl-3e-=Al3+��
�����缫ͬʱ����Ũ����ʱ��������ָ��ƫ��ͭ���������ͭ����������ʱ�������������������������Ƭ�ϵ缫��ӦʽΪ2NO3-+2e-+4H+=2NO2��+2H2O��
��3����Դ���������ǹ��ĵ��ȵ㣬��������ý�������ȼ�ϣ�������һ�ִ���ӱ�����룮�Դˣ���Ĺ۵���B���A����B�������������ǹ�ҵ���õ���������ķ�����ȡ����Ҫ���Ĵ����ĵ��ܣ�A������B�������У�
��������������й�ʵ���������£�
| �۵�/�� | �е�/�� | ȼ����/kJ•mol-1 | |
| �� | 660 | 2467 | 602 |
| ������ | 2050 | 2980 | / |
��2������Լ6cm����2cm��ͭƬ����Ƭ��һƬ���ֱ��ý�����ƽ�еع̶���һ�����ϰ��ϣ����2cm������ͭƬ����Ƭ�ֱ�͵������ġ�+������-���������ӣ�������ָ������м�λ�ã�ȡ����50mL��С�ձ�����һ���ձ���ע��Լ40mL��Ũ���ᣬ����һֻ�ձ���ע��40mL0.5mol/L��������Һ���Իش��������⣺
�����缫ͬʱ����ϡ�����У�������ָ��ƫ�������������ͭ����������Ƭ�ϵ缫��ӦʽΪAl-3e-=Al3+��
�����缫ͬʱ����Ũ����ʱ��������ָ��ƫ��ͭ���������ͭ����������ʱ�������������������������Ƭ�ϵ缫��ӦʽΪ2NO3-+2e-+4H+=2NO2��+2H2O��
��3����Դ���������ǹ��ĵ��ȵ㣬��������ý�������ȼ�ϣ�������һ�ִ���ӱ�����룮�Դˣ���Ĺ۵���B���A����B�������������ǹ�ҵ���õ���������ķ�����ȡ����Ҫ���Ĵ����ĵ��ܣ�A������B�������У�
18�����в��������ʵ��ǣ�������
| A�� | ������Ӧ����ˮԡ���� | |
| B�� | ��ͨ��ʢ��ˮ��ϴ��ƿ�ķ�����ȥ���������к��е���ϩ���� | |
| C�� | ����ȩ��ԭ����Cu��OH��2����Һ��ʵ���У���Cu��OH��2����ҺʱӦ����NaOH��������ֱ�Ӽ��� | |
| D�� | ����������Һʱ����AgNO3��Һ���백ˮ�� |
19������˵����ȷ���ǣ�������
| A�� | ��${\;}_{1}^{2}$H��${\;}_{8}^{18}$O����ɵ�11gˮ������������Ϊ6NA | |
| B�� | H2O��D2O����Ϊͬ�������壬�����ߵĻ�ѧ�������� | |
| C�� | ${\;}_{8}^{18}$O2��${\;}_{8}^{16}$O3����Ϊͬλ�� | |
| D�� | ���ʯ��ʯī��Ϊͬ�������壬����֮���ת�����������仯 |