��Ŀ����
����Ŀ��Ϊ�˲ⶨijþ���Ͻ�ijɷ֣�ȡ14.7 g�Ͻ���ȫ����500 mL 3 mol/L�������У��ټ���400 mL 8 mol/L������������Һ��ַ�Ӧ�����ֻ����һ�ֳ���������ڸúϽ�IJⶨ���̵�������ȷ����
A.�úϽ��к���������������Ϊ5.4 g
B.�Ͻ���þ����������Ϊ63.3%��Mg%<100%
C.�ڲ������������Һ��һ������0.2 mol NaAlO2
D.�ڲ������������Һ����1.5 mol Na2SO4
���𰸡�BD
��������
14.7gþ���Ͻ���ȫ����500mL 3mol/L�������У��ټ���400mL 8mol/L������������Һ��Ӧ����Һ�еij��������ӣ�����٣���þ������ǡ�ó�����������þ����������ʱ����Һ��ֻ����Na2SO4����ʱn(Na+)=2n(SO42)����Ϊ��n(Na+)=n(NaOH)��n(SO42)=n(H2SO4)������n(NaOH)=2n(H2SO4),�������ɵij��������ʱ��n(NaOH)=2n(H2SO4)=2��3mol/L��0.5L=3mol��������400mL 8mol/L������������Һ��ַ�Ӧֻ����һ�ֳ�����֤�����������Ѿ�������������ȫ��Ӧ��������������Ϊ��0.4L��8mol/L=3.2mol���ܽ���������������������Ϊ��3.2mol3mol=0.2mol
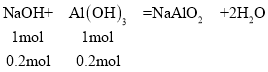
�����������������Ϊ0.2mol����ԭ���غ�֪��n(Al)=n[Al(OH)3]=0.2mol��m(Al)=0.2mol��27g/mol=5.4g��m(Mg)=14.7gm(Al)=14.75.4g=9.3g��
A�����Ϸ���֪���úϽ��к���������������Ϊ5.4g����A����
B�����Ϸ���֪���úϽ��������������Ϊ5.4g��þ������Ӧ����9.3g14.7g֮��(����9.3g)��![]() ��100%Mg%��
��100%Mg%��![]() ��100%����63.3%Mg%��100%����B��ȷ��
��100%����63.3%Mg%��100%����B��ȷ��
C�����Ϸ���֪���úϽ��к���������������Ϊ0.2mol���ڲ������������Һ����ຬ��0.2mol NaAlO2����C����
D���ڲ������������Һ����Na2SO4��NaAlO2������ԭ���غ�֪��n(Na2SO4)=n(H2SO4)=3mol/L��0.5L=1.5mol����D��ȷ��
��ѡBD��

����Ŀ��һ�������£���2L�����ܱ������г���1mol PCl5��������Ӧ��PCl5(g)![]() PCl3(g)+Cl2(g)��Ӧ�����вⶨ�IJ������ݼ�������Ӧ�������������䣩��
PCl3(g)+Cl2(g)��Ӧ�����вⶨ�IJ������ݼ�������Ӧ�������������䣩��
t/s | 0 | 60 | 150 | 250 | 350 | 450 |
n(PCl3)/mol | 0 | 0.12 | 0.19 | 0.2 | 0.2 | x |
��ش��������⣺
��1��x��ֵ��___��
��2��0~60s�ڣ���PCl3��Ũ�ȱ仯��ʾ�Ļ�ѧ��Ӧ������__��
��3��60sʱ��PCl5��ת������__��
��4����ƽ��ʱ��������Cl2�����������__���������һλС�������ɱ������ݼ���������£��÷�Ӧ��ƽ�ⳣ����__mol��L-1��