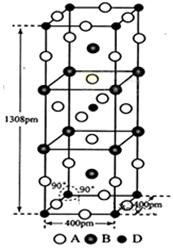
��Ŀ����
A��B��C��D��E���ֶ�����Ԫ��,��֪:
��ԭ�Ӱ뾶����˳��Ϊ:A��D��C��B��E
�ڶ�����(��ϡ������Ԫ����)����Ԫ����A��ԭ�Ӱ뾶��E��ԭ�Ӱ뾶֮��Ϊ��С
��B��C��D����Ԫ�ص��Ӳ���ͬ,����ԭ������֮��Ϊ21,��Dԭ�Ӵ���������Ϊ������������,
��ش���������:
(1)д��E������D������ȼ�ղ���ĵ���ʽ:����������������������
(2)C2A4��H2O��NH3��H2O����,ˮ��ҺҲ��������,�õ��뷽��ʽ��ʾ��ˮ��Һ�������Ե�ԭ��:��������������������������
(3)B��D��E����Ԫ�ؿ���ɳ���������X,A��B��D��E����ɳ���������Y,X��Y���������г����Ļ�ѧ�Լ�,������һ�������¿ɻ���ת������a mol Yת����a mol Xʱ:(��Һ�н���)
��������a mol������Z�Ϳ�ʵ��ת��,�����ZΪ��������(��һ��Z���ʻ�ѧʽ)��
��������0.5a mol������Z�Ϳ�ʵ��ת��,�����ZΪ��������(��һ��Z���ʻ�ѧʽ)��
(4)C2A4����������ȼ�ϵ�ص�ȼ��,������֮һΪC�ĵ���,���Բ������缫,KOH���������Һ����ԭ���,д�������ĵ缫��Ӧ:����������������������;�øõ�ص�����CuSO4��Һ,���ռ���3.36 L(��״��)������,�������6.4 g C2A4,���ȼ�ϵ�������Ϊ��������������
(1)Na+[��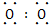 ��]2-Na+
��]2-Na+
(2)N2H4��H2O N2
N2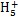 +OH-
+OH-
(3)��NaOH(������������)����Na2O(������������)
(4) N2H4-4e-+4OH- N2+4H2O��75%
N2+4H2O��75%
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�������ѧ��ѧ����Ԫ��ԭ�ӽṹ�����������ʾ��
| ��� | Ԫ�� | �ṹ������ |
| �� | A | A�����������г������������������Ȼ����Է����������35.5 |
| �� | B | Bԭ���������������ڲ���������� |
| �� | C | C�dz������ʵ���ҪԪ�أ����ʳ����³���̬ |
| �� | D | D���ʱ���Ϊ����Ϣ�����Ĵ��������dz��õİ뵼����� |
| �� | E | ͨ������£�Eû�������ϼۣ�A��B��C��D��F������E�γɻ����� |
| �� | F | F�����ڱ��п������ڢ�A�壬Ҳ����������ڢ�A�� |
��1��Aԭ�������ڱ��е�λ��Ϊ________��
��2��B��C�γɵĻ�����Ļ�ѧʽΪ________��������________���������ӡ����ۡ�����
��3��F��E�����γ�10���Ӻ�18���ӵ����ֻ�����X��Y������X��Y��ˮ��Һ��ʵ�鷽����_______________________________________��
��4��C��E���ǽϻ��õķǽ���Ԫ�أ��û�ѧ����ʽ���������ֵ��ʵ�������ǿ��__________________________________________________________��
��5���о�һ��ʵ����ʵ֤��A��B����Ԫ�ص��ʻ�ԭ�Ե�ǿ��_______________________________________________________________��
W��X��Y��Z��Ԫ�����ڱ���ԭ��ǰ�����ڵ�����Ԫ�أ��й����ǵ���Ϣ���±���ʾ��
| Ԫ�� | �����Ϣ |
| �� | W�Ļ�̬ԭ��L���������K���������3�� |
| �� | X����Ԫ���γɵ�һ�ֻ��������ʹʪ��ĺ�ɫʯ����ֽ���� |
| �� | ����Ϊ����ɫ���壬����������Ӧ��ˮ���ﻯѧʽΪH��O4 |
| �� | Z�Ļ�̬ԭ����Χ�����Ų�ʽΪ(n��1)d10ns1 |
��1��Yλ��Ԫ�����ڱ���________���ڵ�________�壻X�Ļ�̬ԭ�Ӻ�������Ų�ʽ�� ��
��2��W�ļ����Ӱ뾶 X�ļ����Ӱ뾶���>������<����=������Y�ĵ�һ�����ܱ�Z�� �����С������W��X�������̬�⻯���У��е�ϸߵ��� ���ѧʽ����
��3����150������ʱ��������ZW��������Ӧ���ɺ�ɫ��Z2W��ĩ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4��WԪ���γɵĶ��ֻ���������У����м��Թ��ۼ��ͷǼ��Թ��ۼ��ķ�������Ϊ
����дһ�֣���
��5����25�桢101 kPa�£���֪Z���嵥����Y2��������ȫȼ�պ�ָ���ԭ״̬��ƽ��ÿת��1mol ���ӷ���110.05kJ���÷�Ӧ���Ȼ�ѧ����ʽ�� ��

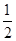 C2��g��=AC2��g������H����283.0 kJ��mol��1
C2��g��=AC2��g������H����283.0 kJ��mol��1