��Ŀ����
W��X��Y��Z��Ԫ�����ڱ���ԭ��ǰ�����ڵ�����Ԫ�أ��й����ǵ���Ϣ���±���ʾ��
| Ԫ�� | �����Ϣ |
| �� | W�Ļ�̬ԭ��L���������K���������3�� |
| �� | X����Ԫ���γɵ�һ�ֻ��������ʹʪ��ĺ�ɫʯ����ֽ���� |
| �� | ����Ϊ����ɫ���壬����������Ӧ��ˮ���ﻯѧʽΪH��O4 |
| �� | Z�Ļ�̬ԭ����Χ�����Ų�ʽΪ(n��1)d10ns1 |
��1��Yλ��Ԫ�����ڱ���________���ڵ�________�壻X�Ļ�̬ԭ�Ӻ�������Ų�ʽ�� ��
��2��W�ļ����Ӱ뾶 X�ļ����Ӱ뾶���>������<����=������Y�ĵ�һ�����ܱ�Z�� �����С������W��X�������̬�⻯���У��е�ϸߵ��� ���ѧʽ����
��3����150������ʱ��������ZW��������Ӧ���ɺ�ɫ��Z2W��ĩ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4��WԪ���γɵĶ��ֻ���������У����м��Թ��ۼ��ͷǼ��Թ��ۼ��ķ�������Ϊ
����дһ�֣���
��5����25�桢101 kPa�£���֪Z���嵥����Y2��������ȫȼ�պ�ָ���ԭ״̬��ƽ��ÿת��1mol ���ӷ���110.05kJ���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��1���� ��A��2��) 1s22s22p3 ��2��)
��2��< ��1��) ��1��) H2O ��2��)
��3��2CuO �� H2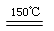 Cu2O �� H2O ��2��)
Cu2O �� H2O ��2��)
��4���������⣨˫��ˮ����2��)
��5��Cu(s) ��Cl2(g)��CuCl2(s) ��H=��220.10kJ/mol��3��)
�������������W�Ļ�̬ԭ��L���������K���������3������WΪOԪ�أ�X����Ԫ���γɵ�һ�ֻ��������ʹʪ��ĺ�ɫʯ����ֽ��������XΪNԪ�أ�Y�ĵ���Ϊ����ɫ���壬����������Ӧ��ˮ���ﻯѧʽΪH��O4����YΪClԪ�أ�Z�Ļ�̬ԭ����Χ�����Ų�ʽΪ(n��1)d10ns1����Ϊ������Ԫ��λ��Ԫ�����ڱ���ǰ�����ڣ�����ZΪCuO��
��1��YΪClԪ�أ�λ��Ԫ�����ڱ��������ڡ���A�壻XΪNԪ�أ����̬ԭ�Ӻ�������Ų�ʽΪ��1s22s22p3
��2��O2?��N3?�����Ų���ͬ��ԭ������Խ�����Ӱ뾶ԽС������O2?�뾶<N3?�뾶��YΪClԪ�أ�Ϊ���÷ǽ���Ԫ�أ�ZΪCuԪ�أ�Ϊ����Ԫ�أ�����Cl�ĵ�һ�����ܴ���Cu�ĵ�һ�����ܣ�W��X�������̬�⻯��ֱ�ΪH2O��NH3�������£�H2O��Һ���NH3�����壬����H2O�ķе�ߡ�
��3����150������ʱ��������ZW��������Ӧ���ɺ�ɫ��Z2W��ĩ��H2��CuO��ԭΪCu2O����ѧ����ʽΪ��2CuO �� H2 Cu2O �� H2O
Cu2O �� H2O
��4����Ԫ���γɵĻ������У����м��Թ��ۼ��ͷǼ��Թ��ۼ��ķ��ӣ������Ϊ�������⡣
��5������д����ѧ����ʽ��ע�����ʵ�״̬��ÿת��1mol ���ӷ���110.05kJ����1molCu��ȫ��Ӧ���ȣ�220.10kJ������д���Ȼ�ѧ����ʽΪ��Cu(s) ��Cl2(g)��CuCl2(s) ��H=��220.10kJ/mol
���㣺���⿼��Ԫ�ص��ƶϡ�Ԫ�������ɺ�Ԫ�����ڱ�����ѧ����ʽ���Ȼ�ѧ����ʽ����д��

X��Y��Z��W��Ԫ�����ڱ���ԭ������������������ֶ�����Ԫ�أ��������Ϣ���±���
| Ԫ�� | �����Ϣ |
| X | X������������Ӧ��ˮ���ﻯѧʽΪH2XO3 |
| Y | Y�ǵؿ��к�����ߵ�Ԫ�� |
| Z | Z�Ļ�̬ԭ�����������Ų�ʽΪ3s23p1 |
| W | W��һ�ֺ��ص�������Ϊ28��������Ϊ14 |
(1)Wλ��Ԫ�����ڱ���________���ڵ�________�壻W��ԭ�Ӱ뾶��X��________(���С��)��
(2)Z�ĵ�һ�����ܱ�W��________(���С��)��XY2�ɹ�̬��Ϊ��̬����˷���������������________����Ԫ�ء�X��Y��ԭ�ӿɹ�ͬ�γɶ��ַ��ӣ�д������һ�����γ�ͬ�ַ��Ӽ��������������________��
(3)���£���Z���������ᷴӦ�����ɫ��Һ�еμ�NaOH��Һֱ���������ܹ۲쵽��������________��W�ĵ���������ᷴӦ����������ɫ���壬�÷�Ӧ�Ļ�ѧ����ʽ��_________________________________________________��
X��Y��Z��W���ֳ���Ԫ�أ�����X��Y��ZΪ������Ԫ�ء��й���Ϣ���±���
| | ԭ�ӻ���������Ϣ | ���ʼ��仯���������Ϣ |
| X | ZX4�������ɴ�Z�ᴿZ���м���� | X������������Ӧ��ˮ����Ϊ��������ǿ�� |
| Y | Yԭ�ӵ��������������ڵ��Ӳ��� | Y���������ǵ��͵��������������������һ�ּ���ǰ;�ĸ��²��� |
| Z | Zԭ�ӵ������������Ǵ�����������1/2 | Z�����ǽ������ϵ����ǣ��䵥������ȡ���ģ���ɵ�·����Ҫԭ�� |
| W | Wԭ�ӵ�����������С��4 | W�ij������ϼ���+3��+2��WX3ϡ��Һ�ʻ�ɫ |
��1��W�����ڱ���λ��Ϊ ��W��OH��2�ڿ����в��ȶ������ױ��������ɰ�ɫѸ�ٱ�ɻ���ɫ������ɺ��ɫ����Ӧ�Ļ�ѧ����ʽΪ ��
��2��X�ļ������ӵĽṹʾ��ͼΪ ��X������������Ӧˮ�����ˮ��Һ��Y�������ﷴӦ�����ӷ���ʽΪ ��
��3��Z����������ͨѶ���������� ����ҵ���Ʊ�Z�ĵ��ʵĻ�ѧ��Ӧ����ʽΪ ������Z��ͬһ����Ԫ�أ��Ž��з���Ԥ������һԪ�صĴ��ڣ�����������뵼�徧��ܣ������о��������л���������ԵĿ��������ԣ����NaOH ��Һ��Ӧ������H2O2����ʱ����NaOH ��Һ��Ӧ���������Σ��䷽��ʽΪ ��
��4����50 mL l mol��L-1��YX3��Һ����μ���0��5 mol��L-1��NaOH��Һ���õ�1��56 g�����������NaOH��Һ��������� ���������һ�������
 Y2O3(g)����ƽ�ⳣ������ʽΪK= ��
Y2O3(g)����ƽ�ⳣ������ʽΪK= ��

 HCNO + NH3������ƽ�������������գ�
HCNO + NH3������ƽ�������������գ� 2N2��g����3H2O��g��
2N2��g����3H2O��g��