��Ŀ����
��10�֣� A��B��C��D��E��λ�ڶ����ڵ�����Ԫ�ء���֪�������ȶ��ԣ�HmD��HmC����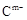 ��
��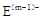 ������ͬ�ĵ��Ӳ�ṹ����A��B��ͬһ���ڣ��ڸ�������������Ԫ���У�A��ԭ�Ӱ뾶���B�����Ӱ뾶��С����A��B������֮����D��������3��������������Ϣ����Ӧ�Ļ�ѧ����ش��������⣺
������ͬ�ĵ��Ӳ�ṹ����A��B��ͬһ���ڣ��ڸ�������������Ԫ���У�A��ԭ�Ӱ뾶���B�����Ӱ뾶��С����A��B������֮����D��������3��������������Ϣ����Ӧ�Ļ�ѧ����ش��������⣺
HmDm�ĵ���ʽ___________________����1�֣�
��֤��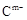 ��
�� �Ļ�ԭ��ǿ�������ӷ���ʽΪ__________________________________��
�Ļ�ԭ��ǿ�������ӷ���ʽΪ__________________________________��
��3����E�ĵ���ͨ��A��D�γɵĻ������ˮ��Һ�У������ӷ���ʽΪ��__________________________��
��4�������£��������ʵ���Ũ�ȵ�HmC��Һ��A������������Ӧ��ˮ������Һ�������ϣ�д���÷�Ӧ�����ӷ���ʽ ��
�ڸ���Һ�������к��еĻ�ѧ�������� ��1�֣�
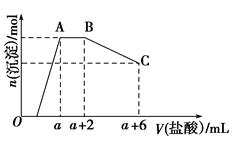
��5����A��B��C��E�����У���������ת����ϵ����_____________����Ԫ�ط��ţ���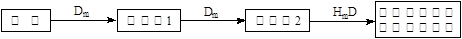
��10�֣�ÿ��2�֣�
��1�� 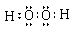 ��1�֣�
��1�֣�
��2�� Cl2 + S2��= 2Cl��+ S��
��3�� Cl2 + 2OH��= Cl��+ ClO��+ H2O
��4�� H2S+ OH��= HS��+ H2O ���Ӽ����ۼ���1�֣�
��5�� Na��S
���������������1�����ݢٿ�֪C��D��ͬ����Ԫ�أ�D�ķǽ����Ա�Cǿ������D�ǵڶ����ڣ�C�ǵ�������Ԫ�أ����ݢۢܿ�֪A�ǵ�һ����Ԫ�أ�B�ǵ�������Ԫ�أ��Ҷ��ǵ�������Ԫ�أ�����A ��Na��B��Al����D��O��C��SԪ�أ�����E��ClԪ�ء�HmDm�ĵ���ʽ����������ĵ���ʽΪ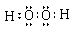 ��
��
��2��S2-�Ļ�ԭ�Ա�Cl-�Ļ�ԭ��ǿ������Ϊ��Cl2 + S2��= 2Cl��+ S�����ӷ�Ӧ������
��3��A��D�Ļ������ˮ��Һ������������Һ��E�ĵ�����������������������������Һ��Ӧ�����ӷ���ʽΪCl2 + 2OH��= Cl��+ ClO��+ H2O��
��4��A������������Ӧ��ˮ������Һ������������Һ��������ʵ�����H2S��Ӧ�������⻯�ƺ�ˮ�����ӷ���ʽΪH2S+ OH��= HS��+ H2O������Һ��������NaHS��������ѧ�������������Ӽ����ۼ���
��5��������Ԫ���п��Է�������������Ԫ����Na��S��Na��������Ӧ���������ƣ��������������������ɹ������ƣ�����������ˮ��Ӧ�����������ƣ���������ͼ��S��������Ӧ���ɶ���������������������һ�������·�Ӧ������������������������ˮ�������ᡣ
���㣺����Ԫ�ص��ƶϣ�Ԫ�ؼ��仯����Ļ�ѧ���ʣ����ӷ���ʽ������ʽ����д����ѧ�����ж�

 ������������ϵ�д�
������������ϵ�д�����ˮ��Һ�д��������һ��������
| A��H+��Fe3+��I����SO42�� |
| B��Al3+��Mg2+��HCO3����Cl�� |
| C��K+��Ca2+��NO3����SiO32�� |
| D��K+��Na+��OH����AlO2�� |
���н���ʵ����ʵ�ķ�Ӧ����ʽ����ȷ����
| A��ʢ���ռ���Լ�ƿ�����ò�������SiO2+2NaOH��Na2SiO3+H2O |
| B�����ռ���Һ����������Cl2+2OH����Cl��+ClO��+H2O |
C����KSCN��Һ����Fe3+��Fe3++3SCN�� Fe(SCN)3 Fe(SCN)3 |
| D������KI������Һ���ú������4I��+O2+2H2O��2I2+4OH�� |
��18�֣��������(K2FeO4)��һ�ּ�������������������һ������Ͷ��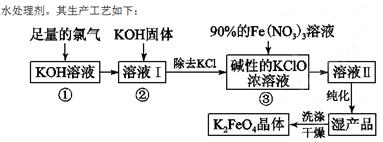
��1����Ӧ��Ӧ���¶Ƚϵ͵�����½��С������¶Ƚϸ�ʱKOH��Cl2��Ӧ���ɵ���KClO3��д�����¶Ƚϸ�ʱKOH��Cl2��Ӧ�Ļ�ѧ����ʽ ���÷�Ӧ������������ ��
��2������Һ���м���KOH�����Ŀ���� �����ţ���
| A������Һ���й�����Cl2������Ӧ�����ɸ����KClO |
| B��KOH�����ܽ�ʱ��ų��϶����������������߷�Ӧ���� |
| C��Ϊ��һ����Ӧ�ṩ���ԵĻ��� |
| D��ʹKClO3ת��ΪKClO |
��4������ж�K2FeO4�����Ѿ�ϴ�Ӹɾ� ��
��5��������أ�K2FeO4����Ϊˮ��������һ���ŵ�������ˮ��Ӧ���ɽ����������ʣ���ƽ�÷�Ӧ�����ӷ���ʽ��
___FeO
 ��____H2O �� ____Fe��OH��3�����壩��____O2����____OH����
��____H2O �� ____Fe��OH��3�����壩��____O2����____OH���� 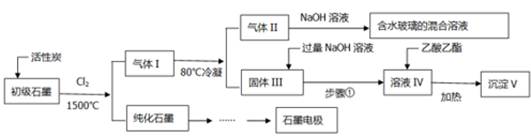

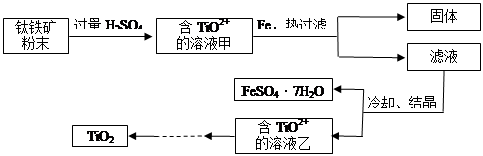
 TiCl4
TiCl4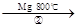 Ti
Ti