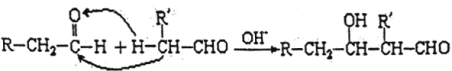
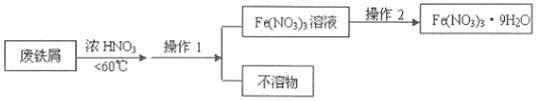
题目内容
【题目】在测定硫酸铜晶体中结晶水含量的实验操作中:
(1)加热前应将晶体放在__________中研碎,加热是放在__________中进行,加热失水后,应放在__________中冷却。
(2)判断是否完全失水的方法是______________________________________________。
(3)下面是某学生一次实验的数据,请完成计算,填入下面的表格中。
坩埚质量 | 坩埚与晶体总质量 | 加热后坩埚与固体总质量 | 测得晶体中结晶水个数 |
11.7 g | 22.7 g | 17. 6 g | _________ |
(4)这次实验中产生误差的原因可能是__________(填写字母)所造成。
A.硫酸铜晶体中含有易挥发性杂志 B.实验前晶体已部分变白
C.加热时固体部分变黑 D.加热失水后漏置在空气中冷却
(5)已知在坩埚中加热硫酸铜晶体,受热分解过程如下:
CuSO4·5H2O![]() CuSO4·3H2O
CuSO4·3H2O ![]() CuSO4·H2O
CuSO4·H2O ![]() CuSO4
CuSO4
有人借助如图封闭装置进行硫酸铜晶体脱水实验,回答下列问题:
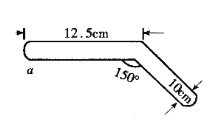
①本实验可用于验证的化学定律是_____________________________。
②a处加热片刻后现象:______________________________________。
③你认为此装置设计是否合理.科学?如不合理,请写出理由:___________________________________________________________________________________________________。
【答案】研钵 坩埚 干燥器 若连续两次加热的质量差不超过0.1 g,则说明完全失水 7.7 ACD 质量守恒定律 蓝色晶体变为白色粉 不合理;封闭仪器不宜加热。
【解析】
硫酸铜晶体中结晶水的质量分数=![]() (结晶水的质量=硫酸铜晶体和瓷坩埚的质量—无水硫酸铜和瓷坩埚的质量 )。步骤为①研磨:在研钵中将硫酸铜晶体研碎。②称量;准确称量干燥的瓷坩埚的质量,并用此坩埚准确称取一定质量已研碎的硫酸铜晶体。③加热:加热晶体,使其失去全部结晶水(由蓝色完全变为白色)。④称量:在干燥器内冷却后称量,并记下瓷坩埚和无水硫酸铜的质量。⑤再加热、再称量至恒重:把盛有无水硫酸铜的瓷坩埚再加热,再放入干燥器里冷却后再称量,记下质量。到连续两次称量的质量相差不超过0.1g为止。⑥计算:根据实验测得的结果求硫酸铜晶体中结晶水的质量分数。
(结晶水的质量=硫酸铜晶体和瓷坩埚的质量—无水硫酸铜和瓷坩埚的质量 )。步骤为①研磨:在研钵中将硫酸铜晶体研碎。②称量;准确称量干燥的瓷坩埚的质量,并用此坩埚准确称取一定质量已研碎的硫酸铜晶体。③加热:加热晶体,使其失去全部结晶水(由蓝色完全变为白色)。④称量:在干燥器内冷却后称量,并记下瓷坩埚和无水硫酸铜的质量。⑤再加热、再称量至恒重:把盛有无水硫酸铜的瓷坩埚再加热,再放入干燥器里冷却后再称量,记下质量。到连续两次称量的质量相差不超过0.1g为止。⑥计算:根据实验测得的结果求硫酸铜晶体中结晶水的质量分数。
(1)加热前应将晶体放在研钵中研碎,加热是放在坩埚中进行,加热失水后,应放在干燥器中冷却。
(2)判断是否完全失水的方法是若连续两次加热的质量差不超过0.1 g,则说明完全失水。
(3)结晶水的质量=硫酸铜晶体和瓷坩埚的质量—无水硫酸铜和瓷坩埚的质量=22.7g-17.6g=5.1g,结晶水的个数=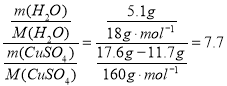 ;
;
(4)A.硫酸铜晶体中含有易挥发性杂志,导致加热过程中固体减少量增多,测定结果偏大,A正确;
B.实验前晶体已部分变白对实验不影响,B错误;
C.加热时固体部分变黑,说明CuSO4已发生分解CuSO4![]() CuO+SO3↑,使测定的结果偏大,C正确;
CuO+SO3↑,使测定的结果偏大,C正确;
D.加热失水后漏置在空气中冷却,固体吸收空气中的水,是固体减少的差值降低,测定结果偏小,D正确。
答案为ACD。
(5)封闭装置进行硫酸铜晶体脱水实验,①实验中物质不会散失,本实验可用于验证的化学定律是质量守恒定律。
②a处加热片刻后,结晶水分解,现象为蓝色晶体变为白色粉;
③此装置不合理,封闭仪器不宜加热。

【题目】将0.20 mol/L的盐酸和物质的量浓度为c mol/L的NaOH溶液按不同体积比配制成两种溶液。下表是配制时所取盐酸与 NaOH 溶液体积与混合后溶液中Na+与Cl-的物质的量浓度数据(忽略溶液体积变化):
溶液 | 混合前所取溶液体积(mL) | 混合后离子浓度(mol/L) | ||
HCl | NaOH | Na+ | Cl- | |
① | 30 | x | 1.5z | z |
② | 10 | y | z | 2z |
下列说法正确( )
A.x=90B.y=30C.z=0.10D.c=0.10