��Ŀ����
19�������½�0.2mol/L HCl��Һ��0.2mol/L MOH��Һ�������ϣ����Ի�Ϻ���Һ����ı仯������û����Һ��pH=6���Իش��������⣺��1���ٻ����Һ����ˮ�������c��H+����HCl��Һ����ˮ�������c��H+�������������������=����
����������Һ��������ʽ�ľ�ȷ����������������֣���
c��Cl-��-c��M+��=9.9��10-7 mol/L��c��H+��-c��MOH��=1��10-8mol/L
��2������������0.2mol/L MOH��Һ��0.1mol/L HCl��Һ�������ϣ���û����Һ��pH��7����˵������ͬ������MOH�ĵ���̶ȣ� MCl��ˮ��̶ȣ������������������=����
��3������������pH=3��HR��Һ��pH=11��NaOH��Һ�������ϣ���û����Һ��pH��7��������Һ��pH��7�������7��������7������ȷ������
���� ��1�������½�0.2mol/L HCl��Һ��0.2mol/L MOH��Һ�������ϣ����Ի�Ϻ���Һ����ı仯��������ǡ�÷�Ӧ����MCl����û����Һ��pH=6����Һ�����ԣ�˵��MOHΪǿ����ʣ�M+����Һ�в���ˮ�⣬��Һ�����ԣ�
�ٻ��Һ��M+����Һ�в���ˮ�⣬�ٽ���ˮ�ĵ��룬��������������������ˮ�ĵ��룻
�ڸ��ݻ��Һ�еĵ���غ㡢�����غ���м��㣻
��2������ϵõ������ʵ���Ũ�ȵ�MCl��MOH�������Һ�����ԣ�˵����ĵ���̶�С���ε�ˮ��̶ȣ�
��3��pH=3��HR��Һ��pH=11��NaOH��Һ��������Һ��c��H+��=c��OH-������HRΪǿ�ᣬ��Ӧ������ԣ���Ϊ���ᣬ��Ӧ������ԣ�
��� �⣺�����½�0.2mol/L HCl��Һ��0.2mol/L MOH��Һ�������ϣ����Ի�Ϻ���Һ����ı仯��������ǡ�÷�Ӧ����MCl����û����Һ��pH=6����Һ�����ԣ�˵��MOHΪǿ����ʣ�M+����Һ�в���ˮ�⣬��Һ�����ԣ�
�ٻ��ҺΪMCl��Һ������M+����Һ�в���ˮ�⣬�ٽ���ˮ�ĵ��룬������������������ˮ�ĵ��룬������Һ����ˮ�������c��H+����HCl��Һ����ˮ�������c��H+����
�ʴ�Ϊ������
�ڻ��Һ��pH=6����c��H+��=1��10-6mol/L��c��OH-��=1��10-8mol/L�����ݵ���غ�c��Cl-��+c��OH-��=c��M+��+c��H+����֪��c��Cl-��-c��M+��=c��H+��-c��OH-��=1��10-6mol/L-1��10-8mol/L=9.9��10-7 mol/L��
���������غ�ɵã�c��H+��-c��MOH��=c��OH-��=1��10-8 mol/L��
�ʴ�Ϊ��9.9��10-7��
��2������ϵõ������ʵ���Ũ�ȵ�MCl��MOH�������Һ�����ԣ�˵����ĵ���̶�С���ε�ˮ��̶ȣ�
�ʴ�Ϊ������
��3��HR��Ϊǿ����ʣ����߶���һԪ����������Ϻ���Һ��pH=7���������Һ��pH��7��˵��HRΪ������ʣ�����Ϻ���Һ�����������Ӧ����Һ��ʾ���ԣ���ҺpH��7��
�ʴ�Ϊ����7��
���� ���⿼��������ϵĶ����жϼ��й�pH�ļ��㣬��Ŀ�Ѷ��еȣ�ע�����յ���غ㡢�����غ㡢�����غ�ĺ��弰Ӧ�÷�������ȷ����ϵĶ����жϷ�������ҺpH�ļ��㷽����

| A�� | �������� | B�� | ���� | C�� | ������ | D�� | ���� |
| A�� | 2-��-2-��ϩ | B�� | 2-��ϩ | C�� | 1-��ϩ | D�� | ��ϩ |
| A�� | ��ˮ�ܸ��Ȼ�������Һ��Ӧ�������������� | |
| B�� | ��������ֽ� | |
| C�� | 0.1mol/L�İ�ˮ����ʹ��̪��Һ��� | |
| D�� | 0.1mol/L��NH4Cl��Һ��pHԼΪ5 |
| A�� | KOH | B�� | K2CO3 | C�� | ZnSO4 | D�� | Zn��OH��2 |

�����й�˵������ȷ���ǣ�������
| A�� | X�Լ�����Na2SO3������Һ | |
| B�� | ���������ӷ�Ӧ��2Br-+Cl2�T2Cl-+Br2 | |
| C�� | ��ҵ��ÿ���1molBr2����Ҫ����Cl244.8L | |
| D�� | �����������ȡ����Һ������ |
| A�� | c��HCOO-����c��Na+�� | B�� | c��HCOOH����c��HCOO-�� | ||
| C�� | c��HCOOH��+c��HCOO-��=0.02mol•L-1 | D�� | 2c��H+��+c��HCOOH��=c��HCOO-��+2c��OH-�� |

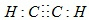 ��A�Ķ���ȡ������1�֣�
��A�Ķ���ȡ������1�֣�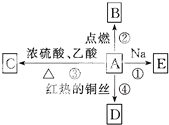 A�ǻ�ѧʵ������������л���ܽ�����ͼ��ʾ�Ķ��ַ�Ӧ��
A�ǻ�ѧʵ������������л���ܽ�����ͼ��ʾ�Ķ��ַ�Ӧ�� CH3COOCH2CH3+H2O����Ӧ��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
CH3COOCH2CH3+H2O����Ӧ��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��