��Ŀ����
25��ʱ��0.1mol Na2CO3�����������õ�һ�����Ϊ1 L����Һ����Һ�в�������pH�Ĺ�ϵ��ͼ��ʾ�������й���Һ������Ũ�ȹ�ϵ������ȷ���� 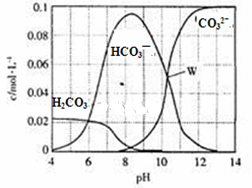
| A��W����ʾ����Һ�У�c (Na+)+c (H+)��2c(CO32- )+c (OH-)+c (Cl-) |
| B��pH= 4����Һ�У�c (H2CO3)+c (HCO3-)+c (CO32-)��0.1mol��L-1 |
| C��pH=8����Һ�У�c (H+)+c (H2CO3)+c (HCO3-)�� c (OH-)+c (Cl- ) |
| D��pH=11����Һ�У�c (Na+)��c (Cl-)��c (CO32- )��c (HCO3- )��c (H2CO3) |
B
�������������A����W��c (HCO3- )= c(CO32- ).���ݵ���غ�ɵã�c (Na+)+c (H+)��2c(CO32- )+c (OH-)+c (Cl-)+ c (HCO3- )��=3c(CO32- )+c (OH-)+c (Cl-)������B����pH= 4����Һ��H2CO3�ᷢ���ֽⷴӦ����CO2�����ݳ������c (H2CO3)+c (HCO3-)+c (CO32-)��0.1mol/L����ȷ��C����pH=8����Һ�У�c (Na+)+c (H+)�� c (OH-)+c (Cl- ) +c (HCO3-)+2c(CO32- )������D��pH=11����Һ�У�c (Na+)��c (CO32- )��c (Cl-)��c (HCO3- )��c (H2CO3)������
���㣺������Һ��pH������Ũ�ȵĴ�С��ϵ��֪ʶ��

 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д��������ʵ�ˮ��Һ������Ũ���������������ܵõ�ԭ���ʵ���( )
| A��FeC13 | B��Ca(HCO3)2 | C��Na[Al (OH)4] | D��Na2SO3 |
���бȽ��У���ȷ����
| A�����µ����ʵ���Ũ����Һ�У�HF��HCN���룬��NaF��Һ��pH��NaCN��Һ�� |
| B��0.2 mol/L NH4Cl��Һ��0.1 mol/LNaOH��Һ�������Ϻ�c(NH4+)��c(Cl��)��c(Na+)��c(OH��)��c(H+) |
| C�����ʵ���Ũ����ȵ�H2S��NaHS�����Һ�У�c(Na+)= c(S2��)+ c(HS��)+ c(H2S) |
| D��ͬŨ�ȵ�������Һ�У���NH4Al(SO4) 2��NH4Cl��CH3COONH4��NH3��H2O��c(NH4+)�ɴ�С��˳���Ǣ٣��ڣ��ۣ��� |
���и���Һ�У��������ʵ���Ũ�ȹ�ϵ��ȷ����
| A��0.1 mol��L��1 Na2CO3��Һ��c(Na+)=2c(HCO3��)+2c(CO32��)+2c(H2CO3) |
| B��0.1 mol��L��1 NH4Cl��Һ��c(NH4+)=c(Cl��) |
| C�����������Һ�м����������ᣬ�õ������Ի����Һ��c(Na+) > c(CH3COO��) > c(H+) > c(OH��) |
| D�������£���pH=2�������pH=12�İ�ˮ�������Ϻ�c(NH4+) > c(Cl��) > c(OH��) > c(H+) |
������Һ������Ũ�ȵĹ�ϵһ����ȷ����
A�� �� �� ��Һ�У� ��Һ�У�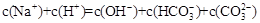 |
B��һԪ����MOH��Ӧ����MCl��Һ�У� |
C�������ʵ�����һԪ����HX�������KX�Ļ����Һ�У� |
D��pH=3��һԪ��HX��pH=11��һԪ��MOH�������ϣ� |
����������������Һ���й�������ȷ����
| | �� | �� | �� | �� |
| pH | 11 | 11 | 3 | 3 |
| ��Һ | ��ˮ | ����������Һ | ���� | ���� |
B���١����зֱ�����������Ȼ�茶��������Һ��pH����С
C���١�������Һ�������ϣ�������Һ��c(NH4+)��c(Cl-)��c(H+)��c(OH-)
D��VaL����VbL����Һ��Ϻ�,����Ϻ���ҺpH=4,��Va��Vb= 9��11
������Һ���й����ʵ�Ũ�ȹ�ϵ��ȷ���� ( )
| A��c��NH4������ȵ�NH4HCO3��NH4HSO4��NH4Cl��Һ�У�c (NH4HSO4) ��c(NH4HCO3) ��c(NH4Cl) |
| B�����������Һ�м����������ᣬ�õ������Ի����Һ�У�C(Na+)��C(CH3COO-)��C(H+)��C(OH-) |
| C��1.0mol/LNa2CO3��Һ�У�C(OH-)=C(HCO3-)+C (H+)+2C(H2CO3) |
| D��ij��Ԫ�������ʽ��NaHA��Һ�У�C(H+)+C(Na+)=C(OH-)+C(HA-)+C(A2-) |
�����£�������������Һ��������ȷ����
| | �� | �� | �� | �� |
| pH | 10 | 10 | 4 | 4 |
| ��Һ | ��ˮ | ����������Һ | ������Һ | ���� |
B���ڡ�������Һ��ȣ����ߵ�kw��ͬ
C���١��ڡ����зֱ���������Ĵ���粒����������Һ��pH����С
D���١�������Һ��һ������Ȼ�ϣ�������Һ������Ũ��˳��һ��Ϊ�� c(NH4+)��c(Cl��)��c(H+)�� c(OH��)
��25��ʱ��pH��11 ��NaOH ��Һ��pH��3 ��CH3COOH��Ҵ�������Ϻ����й�ϵʽ����ȷ����
| A��c (Na+)=c(CH3COO��)+c(CH3COOH) |
| B��c(H+)=c(CH3COO��)+c(OHһ) |
| C��c (Na+) > c (CH3COO��)>c(OH��)>c(H+) |
| D��c (CH3COO��)>c(Na+)>c(H+)>c(OH��) |