��Ŀ����
���бȽ��У���ȷ����
| A�����µ����ʵ���Ũ����Һ�У�HF��HCN���룬��NaF��Һ��pH��NaCN��Һ�� |
| B��0.2 mol/L NH4Cl��Һ��0.1 mol/LNaOH��Һ�������Ϻ�c(NH4+)��c(Cl��)��c(Na+)��c(OH��)��c(H+) |
| C�����ʵ���Ũ����ȵ�H2S��NaHS�����Һ�У�c(Na+)= c(S2��)+ c(HS��)+ c(H2S) |
| D��ͬŨ�ȵ�������Һ�У���NH4Al(SO4) 2��NH4Cl��CH3COONH4��NH3��H2O��c(NH4+)�ɴ�С��˳���Ǣ٣��ڣ��ۣ��� |
D
�������������A�����µ����ʵ���Ũ����Һ�У�HF��HCN���룬HF����ǿǿ��F-ˮ��̶�С������µ����ʵ���Ũ����Һ�У�NaF��Һ��pH��NaCN��ҺС��˵������ȷ��B��0.2 mol/L NH4Cl��Һ��0.1 mol/LNaOH��Һ�������Ϻ���Һ�к���Ũ�ȵ�NaCl��NH4Cl��NH3��H2O����c(Cl��)��c(NH4+)��c(Na+)��c(OH��)��c(H+)������˳�����C�����ʵ���Ũ����ȵ�H2S��NaHS�����Һ�У���S�����ʵ�����Na��2��������2c(Na+)= c(S2��)+ c(HS��)+ c(H2S)��˵������D��ͬŨ�ȵ�������Һ�У���NH4Al(SO4)2��NH4+��Al3+�����ˮ�⣬��NH4Cl(�ο���)��CH3COONH4��NH4+��CH3COO-��ٽ�ˮ���NH3��H2OΪ������ʣ����ֵ��룬��c(NH4+)�ɴ�С��˳���Ǣ٣��ڣ��ۣ�����ȷ��
���㣺ˮ��Һ�е���������

�����£���һԪ��HA����Һ��KOH��Һ��������(��������仯)��ʵ���������±���
| ʵ���� | ��ʼŨ��/(mol��L��1) | ��Ӧ����Һ��pH | |
| c(HA) | c(KOH) | ||
| �� | 0.1 | 0.1 | 9 |
| �� | x | 0.2 | 7 |
A��ʵ��ٷ�Ӧ�����Һ�У�c(OH��)��c(K��)��c(A��)��
 mol/L
mol/LB��ʵ��ٷ�Ӧ�����Һ�У�c(K��)��c(A��)��c(OH��)��c(H��)
C��ʵ��ڷ�Ӧ�����Һ�У�c(A��)��c(HA)��0.1 mol/L
D��ʵ��ڷ�Ӧ�����Һ�У�c(K��)��c(A��)��c(OH��)��c(H��)
������Һ������Ũ�ȴ�С��ϵ�������
A��0.1 mol/L��NH4Cl��Һ�У�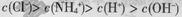 |
B��0.1mol/L��CH3COONa��Һ�У�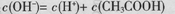 |
C��0.1 mol/LNa2S����Һ�У� |
D��pH=2��������pH=12�İ�ˮ�������Ϻ�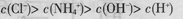 |
����˵���������ȷ����
| A��CH3COONa��Һ�м�������KNO3�����ļ�����Һһ���У� C(Na+)��c(CH3COO��)=c(OH��)��c(H+) |
| B����ZnS��ɫ����Һ�м���CuSO4��Һ���к�ɫ��������������ΪKsp (ZnS)��Ksp (CuS) |
| C������ڵ�NaOH��Һ�еμ�FeCl3������Һ�������Ʊ�Fe(OH)3���� |
| D����ˮ�м���NaCl�ܽ������ͺ���Һ��pH����7 |
��ѧ����ᡢ����������ء��������������ʵ�Ľ�����ȷ����
| ѡ�� | �������ʵ | ���� |
| A | ���ȵ��ռ���Һϴȥ���� | Na2CO3��ֱ�Ӻ����۷�Ӧ |
| B | Ư���ڿ����о��ñ��� | Ư���е�CaCl2 ������е�CO2��Ӧ����CaCO3 |
| C | ʩ��ʱ����ľ��(��Ч�ɷ�ΪK2CO3)������NH4Cl���ʹ�� | K2CO3��NH4Cl��Ӧ���ɰ����ή�ͷ�Ч |
| D | FeCl3��Һ������ͭ��ӡˢ��·������ | FeCl3�ܴӺ���Cu2������Һ���û���ͭ |
�����£�0.2mol/LһԪ��HA���Ũ�ȵ�NaOH��Һ�������Ϻ�������Һ�в�������ּ�Ũ����ͼ��ʾ������˵����ȷ����
| A��HA��ǿ�� |
| B���û��ҺpH=7 |
| C��ͼ��x��ʾHA��Y��ʾOH-��Z��ʾH+ |
| D���û����Һ�У�c(A-)+c(Y)=c��Na+�� |
�����£�Ũ�Ⱦ�Ϊ0.1mol/L����Һ���ٰ�ˮ�������ᡢ���Ȼ����Һ������˵������ȷ����
| A��c(NH4+)���ۣ��� |
| B��ˮ�������c(H+)���ڣ��� |
| C���ٺ͢ڵ������Ϻ����Һ��c(NH4+)+c(NH3��H2O)��0.05mol/L |
| D���ٺ͢۵������Ϻ����ҺpH��7��c(NH4+)��c(Cl��)��c(OH��)��c(H+) |
25��ʱ��0.1mol Na2CO3�����������õ�һ�����Ϊ1 L����Һ����Һ�в�������pH�Ĺ�ϵ��ͼ��ʾ�������й���Һ������Ũ�ȹ�ϵ������ȷ���� 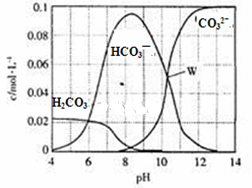
| A��W����ʾ����Һ�У�c (Na+)+c (H+)��2c(CO32- )+c (OH-)+c (Cl-) |
| B��pH= 4����Һ�У�c (H2CO3)+c (HCO3-)+c (CO32-)��0.1mol��L-1 |
| C��pH=8����Һ�У�c (H+)+c (H2CO3)+c (HCO3-)�� c (OH-)+c (Cl- ) |
| D��pH=11����Һ�У�c (Na+)��c (Cl-)��c (CO32- )��c (HCO3- )��c (H2CO3) |
������Һ�е�����Ũ�ȹ�ϵ��ȷ����
| A��0.1 mol/L NaHCO3��Һ�У�c(Na+)��c(HCO3-)��c(CO32-)��c(H2CO3) |
| B��1L0.1 mol/L Na2S��Һ�У�c(OH-)-c(H+)��c(HS-)+c(H2S) |
| C�������£�pH��3.5�ĸ���֭��c(H+)��pH��6.5��ţ����c(H+)��1000�� |
| D��������������ʵ���Ũ�ȵ�NaX������HX��Ϻ����Һ�У� |