��Ŀ����
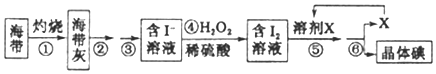
����Ŀ���������ͭ��ؾ���{K2[Cu��C2O4��2]��2H2O������Է�������Ϊ354��������ˮ�;ƾ��������ڰ�ˮ���ڸ���Ļ����½�Ϊ�ȶ������Ե����Ͳ��ᣨH2C2O4��Ϊԭ���Ʊ��������ͭ��ؾ�����������£�
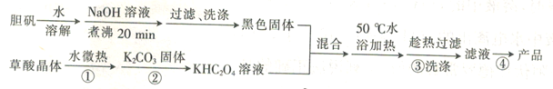
�ش��������⣺
��1��K2[Cu��C2O4��2]��2H2O��Cu�Ļ��ϼ�Ϊ___________��
��2���ڳ����£�������Һ������������Һ��Ӧ����������ͭ����������Һ��pH��7ʱ������Һ��c��Cu2������_________{��֪���¶���Ksp[Cu��OH��2]��2.2��10��20}��
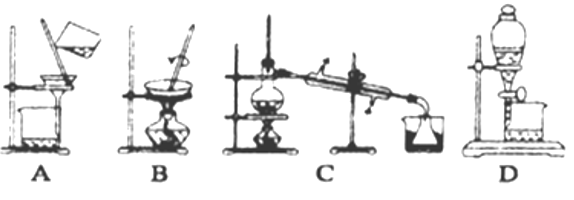
��3����ɫ����Ļ�ѧʽΪ__________________������ʱ�õ��IJ���������©����__________��
��4�����ᾧ�������ֽ⣬����ΪCO��CO2��H2O���÷�Ӧ�Ļ�ѧ����ʽΪ_____________��
��5�������ܺ�õ��ľ������������ƾ�ϴ�ӣ���Ŀ����_________��
��6����Ʒ�Ķ���ȷ��ȡ2.000 g��Ʒ���ڰ�ˮ�У������250 mL��Һ����ȡ25.00 mL��Һ����ƿ�У��ټ���10 mL 3.000 mol��L��1ϡ���ᣬ��0.01000 mol��L��1KMnO4��Һ�ζ���
��������KMnO4��Һ�����Ϊ20.00 mL����ò�Ʒ�Ĵ�����__________��
�ڵζ����������ʢװ��Һ�ĵζ���û����ϴ�����õĽ����_______������ƫ��������ƫ����������Ӱ��������
���𰸡�+2 ![]() CuO �ձ���������
CuO �ձ��������� ![]() ��ȥ����Ŀ��������ʺ�ˮ�֣�ͬʱ�ƾ��ӷ����������� 88.5% ƫ��
��ȥ����Ŀ��������ʺ�ˮ�֣�ͬʱ�ƾ��ӷ����������� 88.5% ƫ��
��������
��1�����ݻ��ϼ۷�����з�����
��2��pH=7ʱ��Һ��![]() =1��10-7��
=1��10-7��![]() ���Լ����c��Cu2+����
���Լ����c��Cu2+����
��3��������������������Һ������п���������ͭ���ʺ�ɫ����ΪCuO�����ݹ��˲��������õ��IJ���������
��4������H2C2O4��C�Ļ��ϼ�Ϊ+3�ۣ����ݵ����غ��Ԫ���غ��д������ʽ��
��5���������ھƾ�������Ļ����½�Ϊ�ȶ���
��6���ٸ��ݵ�ʧ�����غ��֪n(MnO4��)��5=n(C2O42��)��2��(4��3)���ݴ˽��n(C2O42��)������������������ͭ��II��������ʵ��������������ռ������Ʒ�Ĵ��ȣ�
�ڵζ����������ʢװ��Һ�ĵζ���û����ϴ����Һ�ᱻϡ�ͣ��������ñ�Һ������࣬���õĽ����ƫ�ߡ�
��1��K2[Cu��C2O4��2]��2H2O��KΪ+1�ۣ�C2O42-����������ɣ�����Cu�Ļ��ϼ�Ϊ+2�ۣ��ʴ�Ϊ��+2��
��2��pH=7ʱ��Һ��![]() =1��10-7��
=1��10-7��![]() ��֪
��֪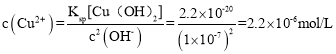 �ʴ�Ϊ��
�ʴ�Ϊ��![]() ��
��
��3��������������������Һ������п���������ͭ���ʺ�ɫ����ΪCuO�������õ��IJ��������У�©�����ձ������������ʴ�Ϊ��CuO���ձ�����������
��4������H2C2O4��C�Ļ��ϼ�Ϊ+3�ۣ����ݵ����غ��Ԫ���غ��֪����ʽΪ��![]() ��
��
��5�����������Ϣ�þ������ھƾ�������Ļ����½�Ϊ�ȶ����ʴ�Ϊ����ȥ����Ŀ��������ʺ�ˮ�֣�ͬʱ�ƾ��ӷ����������
��6���ٸõζ�����ԭ��Ϊ��������������Ի����¿�����������������ݵ�ʧ�����غ��֪n(MnO4��)��5=n(C2O42��)��2��(4��3)�����n(C2O42��)=20.00��10��3��0.01000��![]() mol=5��10��4mol�����������ͭ��II��������ʵ���Ϊ
mol=5��10��4mol�����������ͭ��II��������ʵ���Ϊ![]() mol=2.5��10��4mol����Ʒ�Ĵ���Ϊ
mol=2.5��10��4mol����Ʒ�Ĵ���Ϊ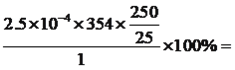 88.5%���ʴ�Ϊ��88.5%��
88.5%���ʴ�Ϊ��88.5%��
�ڵζ����������ʢװ��Һ�ĵζ���û����ϴ����Һ�ᱻϡ�ͣ��������ñ�Һ������࣬���õĽ����ƫ�ߣ��ʴ�Ϊ��ƫ�ߡ�

 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�