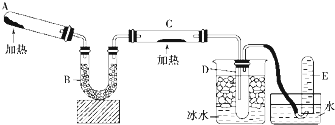
��Ŀ����
4��ijͬѧѧϰ��Ԫ�������ɡ�Ԫ�����ڱ���Ϊ��̽��ͬ����Ԫ�����ʵĵݱ���ɣ��Լ������һ��ʵ�鷽��������¼���й�ʵ�������������������ͬѧ���ʵ�鱨�森��ʵ��Ŀ�ģ�̽��ͬһ����Ԫ�����ʵĵݱ���ɣ�
��ʵ����Ʒ���⻯����Һ���廯����Һ����ˮ����ˮ�����Ȼ�̼��
��������1���Թܣ���2����ͷ�ιܣ�������д������Ҫ�IJ���������
��ʵ�����ݣ�
| ��� | ʵ�鷽�� | ʵ������ |
| �� | ��������ˮ����ʢ������KBr��Һ���Թ��У����ٵ����������Ȼ�̼���� | ������ˮ����Һ����ɫ��Ϊ��ɫ���������Ȼ�̼��ˮ����ɫ��dz�����Ȼ�̼�㣨�²㣩��Ϊ�Ⱥ�ɫ |
| �� | ��������ˮ����ʢ������NaI��Һ���Թ��У����ٵ����������Ȼ�̼���� | ������ˮ����Һ����ɫ��Ϊ��ɫ���������Ȼ�̼��ˮ����ɫ��dz�����Ȼ�̼�㣨�²㣩��Ϊ��ɫ |
������������ۣ�
�����ýṹ���ۼ�˵���ó��������۵�ԭ��4��ԭ�Ӱ뾶Խ��õ�������Խ��
������F2��ͬNa���ڻ��ã��������һ�����û���Ӧʵ����֤��������ǿ�������о�һ����ʵ˵��F�ķǽ����Ա�Clǿ����5���ֱ���������Ӧ��F2�����ף�
���� ��1��ʵ�����Թ��ڽ��У�
��2����ȡҺ��ʱ�ý�ͷ�ιܣ�
��3�������ܽ������Ӵ���ˮ��Һ���û����������嵥���ܽ������Ӵ���ˮ��Һ���û�������˵��������������ǿ���嵥�ʣ��嵥��ǿ�ڵⵥ�ʣ�
��4����ԭ�����������ӣ�ԭ�Ӱ뾶������ԭ�Ӻ˶��ں�����ӵ�������ǿ��
��5����������ӦԽ���ף��ǽ�����Խǿ��
��� �⣺��1��ʵ�����Թ��ڽ��У��ʴ�Ϊ���Թܣ�
��2����ȡҺ��ʱ�ý�ͷ�ιܣ��ʴ�Ϊ����ͷ�ιܣ�
��3�������ܽ������Ӵ���ˮ��Һ���û����������嵥���ܽ������Ӵ���ˮ��Һ���û�������˵��������������ǿ���嵥�ʣ��嵥��ǿ�ڵⵥ�ʣ�Ԫ�صķǽ�����Խǿ�����ǽ����ԣ�Cl��Br��I���ʴ�Ϊ��Cl��Br��I��
��4��ͬһ����Ԫ�أ����϶��£���ԭ�����������ӣ�ԭ�Ӱ뾶������ԭ�Ӻ˶��ں�����ӵ�������ǿ��ԭ��ʧ���������������ʴ�Ϊ��ԭ�Ӱ뾶Խ��õ�������Խ����
��5����������ӦԽ���ף��ǽ�����Խǿ���ֱ���������Ӧ��F2�����ף��ʴ�Ϊ���ֱ���������Ӧ��F2�����ף�
���� ���⿼��ͬһ�������ʵݱ���ɣ�Ϊ��Ƶ���㣬���ؿ���ѧ��ʵ���������������������֪���ǽ�����ǿ���жϷ�������Ŀ�ѶȲ���

| A�� | �ڢ�A��Ԫ�ض��ǽ���Ԫ�� | |
| B�� | �ڢ�A��ǽ���Ԫ�صĵ���ֻ���γ�ԭ�Ӿ��� | |
| C�� | �ڢ�A��Ԫ���γɵĵ��ʣ�������������Һ��������״̬ | |
| D�� | Ԫ�����ʳ��������Ա仯�ĸ���ԭ����Ԫ��ԭ���������������������Ա仯 |
| A�� | 1��3 | B�� | 3��1 | C�� | 1��4 | D�� | 1��1 |
| A�� | ̼��Ƶμ����CO32-+2H+�TCO2+H2 O | |
| B�� | �������������ᷴӦ��H++OH-�TH2O | |
| C�� | ������ͭ�����ᷴӦCu��OH��2+2H+�TCu2++2H2O | |
| D�� | ��Ƭ�������ṯ��Һ�У�Al+Hg2+�TAl3++Hg |